
#40 2C-T-2
2,5-DIMETHOXY-4-ETHYLTHIOPHENETHYLAMINE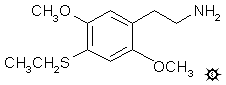
|
|
[3D .jpg image] [3D .mol structure] |
The following reaction is also a very vigorous one and must be performed in a well ventilated place. To a solution of 400 mL 25% H2SO4 (V/V) in a beaker at least 2 L in size, there was added 54 g of 2,5-dimethoxybenzenesulfonyl chloride, and the mixture was heated on a steam bath. The yellow crystals of the acid chloride floated on the surface of the aqueous layer. There should be 80 g of zinc dust at hand. A small amount of Zn dust was placed at one spot on the surface of this chapeau. With occasional stirring with a glass rod, the temperature was allowed to rise. At about 60 or 70 °C an exothermic reaction took place at the spot where the zinc was placed. Additional dollups of zinc were added, and each small exothermic reaction site was spread about with the glass stirring rod. Finally, the reaction spread to the entire solid surface layer, with a melting of the acid chloride and an apparent boiling at the H2O surface. The remainder of the 80 g of zinc dust was added as fast as the size of the reaction container would allow. After things subsided again, the heating was continued for 1 h on the steam bath. After the reaction mixture had cooled to room temperature, it was filtered through paper in a Buchner funnel, and the residual metal washed with 100 mL CH2Cl2. The two-phase filtrate was separated, and the lower, aqueous phase was extracted with 2x75 mL CH2Cl2. The addition of 2 L H2O to the aqueous phase now made it the upper phase in extraction, and this was again extracted with 2x75 mL CH2Cl2. The organic extracts were pooled (H2O washing is more trouble than it is worth) and the solvent removed under vacuum. The light amber residue (30.0 g) was distilled at 70-80 °C at 0.3 mm/Hg to yield 25.3 g 2,5-dimethoxythiophenol as a white oil. This chemical is certainly not centrally active, but it is a most valuable precursor to all members of the 2C-T family.
To a solution of 3.4 g of KOH pellets in 75 mL boiling EtOH, there was added a solution of 10.0 g 2,5-dimethoxythiophenol in 60 mL EtOH followed by 10.9 g ethyl bromide. The reaction was exothermic with the immediate deposition of white solids. This was heated on the steam bath for 1.5 h, added to 1 L H2O, acidified with HCl, and extracted with 3x100 mL CH2Cl2. The pooled extracts were washed with 100 mL of 5% NaOH, and the solvent removed under vacuum. The residue was 2,5-dimethoxyphenyl ethyl sulfide which was a pale amber oil, weighed about 10 g and which was sufficiently pure for use in the next reaction without a distillation step.
A mixture of 19.2 POCl3 and 18.0 g N-methylformanilide was heated briefly on the steam bath. To this claret-colored solution there was added the above 2,5-dimethoxyphenyl ethyl sulfide, and the mixture heated an additional 20 min on the steam bath. This was then added to 500 mL of well-stirred warm H2O (pre-heated to 55 °C) and the stirring continued for 1.5 h by which time the oily phase had completely solidified to a brown sugar-like consistency. The solids were removed by filtration, and washed with additional H2O. After being sucked as dry as possible, these solids were dissolved in 50 mL boiling MeOH which, after cooling in an ice-bath, deposited almost-white crystals of 2,5-dimethoxy-4-(ethylthio)-benzaldehyde. After filtration, modest washing with cold MeOH, and air drying to constant weight, there was obtained 11.0 g of product with a mp of 86-88 °C. Recrystallization of a small sample again from MeOH provided an analytical sample with mp 87-88 °C. Anal. (C11H14O3S) C,H.
To a solution of 11.0 g 2,5-dimethoxy-4-(ethylthio)benzaldehyde in 100 g of nitromethane there was added 0.5 g of anhydrous ammonium acetate, and the mixture was heated on the steam bath for 80 min (this reaction progress must be monitored by TLC, to determine the point at which the starting aldehyde has been consumed). The excess nitromethane was removed under vacuum leaving a residue that spontaneously set to orange-red crystals. These were scraped out to provide 12.9 g crude 2,5-dimethoxy-4-ethylthio-beta-nitrostyrene with a mp of 152-154 °C. A sample recrystallized from toluene was pumpkin colored and had a mp of 148-149 °C. Another sample from acetone melted at 149 °C sharp, and was light orange. From IPA came spectacular fluorescent orange crystals, with a mp 151-152 °C. Anal. (C12H15NO4S) C,H.
A suspension of 12.4 g LAH in 500 mL anhydrous THF was stirred under He. To this there was added 12.4 g 2,5-dimethoxy-4-ethylthio-beta-nitrostyrene in a little THF, and the mixture was held at reflux for 24 h. After the reaction mixture had returned to room temperature, the excess hydride was destroyed by the cautious addition of 60 mL IPA, followed by 20 mL of 5% NaOH followed, in turn, by sufficient H2O to give a white granular character to the oxides. The reaction mixture was filtered, and the filter cake washed first with THF and then with MeOH. Removing the solvents from the combined filtrate and washings under vacuum provided 9.5 g of a yellow oil. This was added to 1 L dilute HCl and washed with 2x100 mL CH2Cl2 which removed all color. After making the aqueous phase basic with 25% NaOH, it was extracted with 3x100 mL CH2Cl2, the extracts pooled, and the solvent removed under vacuum to provide 7.3 g of a pale amber oil. Distillation at 120-130 °C at 0.3 mm/Hg gave 6.17 g of a clear white oil. This was dissolved in 80 mL IPA and neutralized with concentrated HCl, forming immediate crystals of 2,5-dimethoxy-4-ethylthiophenethylamine hydrochloride (2C-T-2). An equal volume of anhydrous Et2O was added and, after complete grinding and mixing, the salt was removed by filtration, washed with Et2O, and air dried to constant weight. The resulting white crystals weighed 6.2 g.
DOSAGE: 12 - 25 mg.
DURATION: 6 - 8 h.
QUALITATIVE COMMENTS: (with 12 mg) I don't feel this for fully an hour, but when I do it is quite a weight. It feels good to work it through. It is OK to be with pain. You can't eliminate it. And it is OK to contact your deep pools of anger. And all of it stems from the lack of acknowledgment. All the macho carrying on, the fights, the wars, are ways of demanding attention, and getting even for not having had it in one's life. I am experiencing more deeply than ever before the importance of acknowledging and deeply honoring each human being. And I was able to go through and resolve some judgments with particular persons.
(with 20 mg) I chose 2C-T-2 at this dose level because the lateness of getting started, and I wanted a shorter experience with my daughter and her family around. I feel, however, that I have somewhat less of a body load with 2C-T-7. Today I was badly in need of the help that might possibly come from this material, and today it was my ally. I sorely needed the type of help that it afforded. The result was to work off the heavy feeling of tiredness and lack of motivation that had been hounding me. The next day I felt that I had dropped my burden.
(with 20 mg) There is a neutralness to this. I am at the maximum, and I am asking myself, 'Am I enjoying this?' And the answer is, 'No, I am experiencing it.' Enjoyment seems beside the point. It is a rather intensely matter-of-fact +3. Is it interesting? Yes, but mostly in expectation of further developments. Is it inspiring? No. Is it negative? No. Am I glad I took it? Yes. Not glad. Satisfied and contented. This is a controlled +3. No threat. The body is all right. Not superbly healthy--but OK. Of no interest, either way. If I were to define the body's state, I would have to define it in image. The image is of a not comfortable state of being clenched. Clenched? Well, carefully bound in control.
(with 22 mg) A slow onset. It took an hour for a plus one, and almost another two hours to get to a +++. Very vivid fantasy images, eyes closed, but no blurring of lines between "reality" and fantasy. Some yellow-grey patterns a la psilocybin. Acute diarrhea at about the fourth hour but no other obvious physical problems. Erotic lovely. Good material for unknown number of possible uses. Can explore for a long time. Better try 20 milligrams next time.
(with 25 mg) I was at a +++ in an hour! It is most difficult to do even ordinary things. I took notes but now I can't find them. This is much too high for anything creative, such as looking at pictures or trying to read. Talking is OK. And to my surprise I was able to get to sleep, and a good sleep, at the seven hour point.
EXTENSIONS AND COMMENTARY: There is a considerable parallel between 2C-T-2 and 2C-T-7, and both have proven to be excellent tools for introspection. The differences are largely physical. With 2C-T-2, there is more of a tendency to have physical disturbances such as nausea and diarrhea. And the experience is distinctly shorter. With 2C-T-7, physical disturbances are less common, but you are into the effects for almost twice as long. Both have been frequently used in therapy as follow-ups to MDMA.
A point of potential misidentification should be mentioned here. 2C-T-2 has occasionally been called, simply, T-2. This abbreviated nickname has also been used for T-2 Toxin, a mycotoxin of the Tricothecene group, formed mainly by the Fusarium spp. This is the infamous "warfare agent" in Southeast Asia, which was finally identified as bee feces rather than a Soviet military adventure. T-2 and 2C-T-2 are radically different compounds.
All three Tweetios of 2C-T-2 have been made and looked at through human eyes. The 2-EtO-homologue of 2C-T-2 is 2-ethoxy-4-ethylthio-5-methoxyphenethylamine, or 2CT2-2ETO. The benzaldehyde (2-ethoxy-4-ethylthio-5-methoxybenzaldehyde) had a melting point of 73-75 °C, the nitrostyrene intermediate a melting point of 122-123 °C, and the final hydrochloride a melting point of 202-204 °C. Fifty milligrams was a completely effective level. The effects were felt very quickly. Vision was blurred, and there were intense eyes-closed visuals and the generation of a pleasant, contemplative mood. Baseline was re-established in five or six hours, but sleep was restless, with weird dreams. Nasal administration showed considerable variation between individuals, but a typical dose was 10 milligrams.
The 5-EtO-homologue of 2C-T-2 is 5-ethoxy-4-ethylthio-2-methoxyphenethylamine, or 2CT2-5ETO. The benzaldehyde (5-ethoxy-4-ethylthio-2-methoxybenzaldehyde) had a melting point of 49 °C, but it was impure. The nitrostyrene intermediate melted at 107-108 °C, and the final hydrochloride had a melting point of 180 °C. At levels of 20 milligrams, there was a slow, gentle climb to a full effect at the third or fourth hour. The flooding of thoughts and easy conversation lasted for many hours, and on some occasion a sedative was needed at the 16 hour point. There was a feeling of being drained for the following day or two. Some intoxication was still noted in the second day. Again it is true here, as had been stated as a generality, that the 5-Tweetio analogues have potencies similar to that of the parent compound, but show a much longer duration. The nickname of "forever yours" had been applied. There may indeed be insight, but 24 hours' worth is an awful lot of insight.
The 2,5-DiEtO-homologue of 2C-T-2 is 2,5-diethoxy-4-ethylthiophen-ethylamine, or 2CT2-2,5DIETO. The benzaldehyde, 2,5-diethoxy-4-(ethylthio)benzaldehyde, had a melting point of 84-85 °C, the nitrostyrene intermediate a melting point of 123-124 °C, and the final hydrochloride a melting point of 220-221 °C. Levels that were evaluated from 10 to 50 milligrams were not particularly different in intensity, but were progressively longer in duration. At 50 milligrams there was a nervousness and edginess during the early part of the experience, but for the next several hours there was evident both energy and high attentiveness. There were few if any sensory alterations. There were no negatives on the following day. The duration was perhaps nine hours.
| [ |
[Main Index] | [Forward |

