
#79 F-2
2-M; 6-(2-AMINOPROPYL)-5-METHOXY-2-METHYL-2,3-DIHYDROBENZOFURAN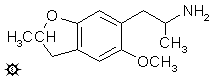
|
| [3D .mol structure] |
A round-bottomed flask containing 93 g crude 4-allyloxyanisole was equipped with an immersed thermometer and heated with an external flame until an exothermic reaction set in at 230 °C. The temperature rose to 270 °C and it was maintained there with the flame for five minutes. After cooling to room temperature, the reaction mix was poured into 2 L H2O and made strongly basic with the addition of 25% NaOH. This dark aqueous phase was washed with 2x200 mL CH2Cl2, and then acidified with HCl. This was then extracted with 2x200 mL CH2Cl2, and the pooled extracts washed first with saturated NaHCO3 and then with H2O. Removal of the solvent under vacuum gave 65.6 g of 2-allyl-4-methoxyphenol as a clear, amber oil. To a solution of 1.66 g of this crude phenol in 5 mL hexane with just enough CH2Cl2 added to effect a clear solution, there was added 1.3 g phenyl isocyanate followed with three drops of triethylamine. An exothermic reaction ensued which spontaneously deposited white crystals. These was removed and hexane washed to give 2-allyl-4-methoxyphenyl N-phenyl carbamate, with a mp of 88-89 °C. The acetate ester, from the phenol and acetic anhydride in pyridine, did not crystallize.
To a solution of 37.7 g 2-allyl-4-methoxyphenol in 125 mL glacial acetic acid there was added 19 g zinc chloride followed with 63 mL concentrated HCl. The mixture was held at reflux temperature for 40 min, then cooled to room temperature, diluted with 300 mL H2O, and extracted with 2x200 mL CH2Cl2. The pooled extracts were washed repeatedly with 8% NaOH until the washings remained basic. Removal of the solvent under vacuum gave a clear pale yellow oil that was distilled at the water pump. A fraction boiling at 150-165 °C was 5-methoxy-2-methyl-2,3-dihydrobenzofuran which weighed 25 g and which was a highly refractive colorless oil. The infra-red spectrum indicated that some small amount of hydroxy group was present, but the NMR spectrum was in complete accord with the benzofuran structure. A higher cut in this distillation gave 4.5 g of a phenolic product tentatively assigned the structure of 4-methoxy-2-propenylphenol. The target dihydrobenzo-furan has also been synthesized from the open-ring o-allyl phenol in acetic acid solution with the addition of a catalytic amount of concentrated H2SO4.
To a half-hour pre-incubated mixture of 69 g POCl3 and 60 g N-methylformanilide there was added 29.0 g 5-methoxy-2-methyl-2,3-dihydrobenzofuran and the mixture was heated on the steam bath for 2 h. The reaction mixture was poured into 1 L H2O, and allowed to stir overnight. The brown gummy solids were removed by filtration, and air dried as completely as possible. These weighed 32 g and were shown by GC on OV-17 to consist of two benzaldehyde isomers in a ratio of 7:2. This was triturated under 18 mL MeOH, and the undissolved solids removed by filtration and washed with 6 mL additional MeOH. The mother liquor and washings were saved. The 17.8 g of dull yellow solids that were obtained were repeatedly extracted with 75 mL portions of boiling hexane (4 extracts were required) and each extract, on cooling, deposited yellow crystals of the major aldehyde. The dried crystals of 6-formyl-5-methoxy-2-methyl-2,3-dihydrobenzofuran were combined (9.5 g) and had a mp of 80-82 °C. The methanol washes saved from above were stripped of solvent, and the sticky, orange solids that remained were enriched in the minor aldehyde isomer (3:2 ratio). Several injections of this crude material into a preparative GC OV-17 column gave sufficient quantities of the "wrong" isomer for NMR characterization. The 2-methyl group was intact (eliminating the possibility of a dihydrobenzopyran isomer) and the ring meta-proton splitting required that the formyl group be in the benzofuran 7-position. This crystalline solid was, therefore, 7-formyl-5-methoxy-2-methyl-2,3-dihydrobenzofuran.
A solution of 9 g of 6-formyl-5-methoxy-2-methyl-2,3-dihydrobenzofuran in 35 mL glacial acetic acid was treated with 6 mL of nitroethane followed with 3.1 g anhydrous ammonium acetate. This mixture was heated on the steam bath for 4 h, diluted with half its volume with warm H2O, and seeded with a bit of product that had been obtained separately. The slightly turbid solution slowly crystallized as it cooled, and was finally held at 0 °C for several h. The deep orange product was removed by filtration, washed with 50% acetic acid, and air dried to constant weight. There was thus obtained 7.0 g 5-methoxy-2-methyl-6-(2-nitro-1-propenyl)-2,3-dihydrobenzofuran with a mp of 89-90 °C from MeOH.
A suspension of 5.0 g LAH in 500 mL of well stirred anhydrous Et2O at a gentle reflux, was treated with a warm, saturated solution of 7.0 g 5-methoxy-2-methyl-6-(2-nitro-1-propenyl)-2,3-dihydrobenzofuran in Et2O added dropwise. The mixture was kept at reflux temperature for 36 h, allowed to stand 2 days, and then the excess hydride destroyed by the cautious addition of 500 mL 6% H2SO4. The phases were separated, and the aqueous phase washed with 2x200 mL CH2Cl2. A total of 125 g potassium sodium tartrate was added to the aqueous phase, and sufficient 25% NaOH added to bring the pH to about 10. This phase was extracted with 3x150 mL CH2Cl2, and the pooled extracts were stripped of solvent under vacuum. The residual oil (4.8 g, amber in color) was dissolved in 300 mL anhydrous Et2O which, upon saturation with anhydrous HCl gas gave a clear solution that suddenly deposited white crystals. The hydrochloride salt of 6-(2-aminopropyl)-5-methoxy-2-methyl-2,3-dihydrobenzofuran weighed 2.3 g and was not satisfactory as a solid derivative, but it appears that the oxalate salt is both nonhygroscopic and quite stable. It (F-2) had a mp of 216-218 °C and it displayed a textbook NMR.
DOSAGE: greater than 15 mg.
DURATION: unknown.
EXTENSIONS AND COMMENTARY: This material, which is certainly a mixture of two diastereoisomeric pairs of racemates since there are two chiral centers present, showed no effects at levels of up to 15 milligrams orally. Doses of 100 mg/Kg were without effects in mice following i.p. injections, although half again this amount proved to be lethal. In rats trained to discriminate LSD from saline, F-2 proved to be about 40 times less potent than the reference compound DOM, requiring some 5 mg/Kg for positive responses. But the human trials were only up to about 0.2 mg/Kg.
This was the prototype compound that was originally put together to justify giving a paper at a marijuana conference in Sweden, in 1968. Although I had never done much with marijuana or with its principal ingredients, I thought maybe I could bend the topic a bit to embrace some potentially active phenethylamines. There is a story of an international conference held in Geneva a few years earlier to discuss the worrisome decrease in the elephant population. A German zoologist invested a full eight-hour day in a summary of his 21 volume treatise on the anatomy and the physiology of the elephant. A French sociologist presented a lively slide show on the mating rituals and rutting behavior of the elephant. And a rabbi from Tel Aviv entitled his talk: "Elephants and the Jewish Problem." My Swedish talk should have been named "Marijuana and the Psychedelic Amphetamines." The memorable story of meeting the chief of the Swedish equivalent of the Bureau of Narcotics, and ending up playing Mozart sonatas in the attic of his home, has been spun out elsewhere in the book.
The original concept was a grand plan to imitate two of the three rings of tetrahydrocannabinol. There is an aromatic ring (with an alkyl group and two oxygens on it) and it is fused to a pyran ring with a couple of methyl groups on it. So, if one were to tie the methyl group at the 4-position of DOM around with a short carbon chain into the oxygen atom at the five position, one could squint and say that the resulting amphetamine was kinda something like an analogue of THC. Thus, the resulting six-membered ring (a pyran) or five-membered ring (a furan) could be peppered with methyl groups at different locations (and up to two per location). If the ring was a five-membered structure, then the parent system would be a benzofuran, and the location of methyl groups on the ring would be indicated by the appropriate numbers following the letter RFS which would stand for "furan". And if it were to be a six-membered ring, the resulting benzopyran would be indicated with a RPS for pyran, and again the methyl group or groups would be indicated by the substitution position. This code would cover all polymethylated homologues with codes that would look like F-22 and P-2234. If any of them showed up with fascinating activities, I would extend methyls to ethyls, and work out some whole new naming code at some future time. An early system, naming this compound 2-M for a methyl group on the 2-position of the furan ring, was abandoned when it became apparent that the pyran world would screw everything up.
The isolation of characterizable quantities of 7-formyl-5-methoxy-2-methyl-2,3-dihydrobenzofuran from the benzaldehyde recipe above gave a fleeting fantasy of a whole new direction that this little project might go. If this unexpected benzaldehyde were to be converted to the corresponding amphetamine, one would have 7-(2-aminopropyl)-5-methoxy-2-methyl-2,3-dihydrobenzofuran. Suddenly here would be a 2,3,5-trisubstituted thing with a ring at the 2,3-position, similar to the still unmade MMDA-4. The temptation to be diverted in this way lasted, fortunately, only a few minutes, and the project was shelved. Someday, when there are buckets of spare time or hosts of eager graduate students, some fascinating chemistry might lie this way, and maybe some fascinating pharmacology, even.
The plain furan analogue, without any methyl groups on it, has been made. Five-methoxybenzofuran formed the 6-formyl derivative (the aldehyde) with a mp of 79-80 °C and from it the nitrostyrene (orange needles, mp 89-91 °C) and the final amphetamine (white solids, as the methane sulfonate, mp 141-144 °C) were prepared in a manner similar to the preparation of F-2 above. In the rat studies, it was three times more potent than F-2, but still some 15 times less potent than DOM. And in initial human trials (of up to 30 milligrams) there were again no effects noted. Naming of this material is easy chemically (6-(2-aminopropyl)-5-methoxy-2,3-dihydrobenzofuran) but tricky as to code. If the numbers that follow the RFS give the location of the methyl groups, then this material, without any such groups, can have no numbers following, and should properly be simply "F." OK, it is "F." The preparation or the attempted preparations of other homologues such as F-23 and F-233 are outlined under the recipe for F-22.
| [ |
[Main Index] | [Forward |

