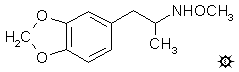
#111 MDMEO
N-METHOXY-MDA; 3,4-METHYLENEDIOXY-N-METHYOXYAMPHETAMINE
SYNTHESIS: To a solution of 20.9 g methoxyamine hydrochloride in 75 mL
MeOH (a strongly acidic solution) there was added 4.45 g
3,4-methylenedioxyphenylacetone (see under MDMA for its preparation)
followed by 1.10 g sodium cyanoborohydride. There was the immediate
formation of a solid phase, and the evolution of what appeared to be
hydrogen cyanide. To this there were added about 4 mL 5% NaOH which
brought the pH to the vicinity of 3 or 4. Another 1.0 g of sodium
cyanoborohydride was added (no gas evolution this time) and stirring
was continued at ambient temperature for 6 days. All was added to 500
mL H2O, acidified with 10 mL HCl, and extraction with 3x100 mL CH2Cl2
removed almost all the color. The aqueous phase was made basic with
25% NaOH, and extracted with 4x100 mL CH2Cl2. Evaporation of the
solvent from these extracts yielded 1.8 g of a pale yellow oil which,
on distillation at 90-95 °C at 0.5 mm/Hg, gave a 1.6 g fraction of an
absolutely white, viscous, clear oil. This was dissolved in 8 mL IPA
and neutralized with concentrated HCl. The product was an
exceptionally weak base, and appropriate end points must be respected
on the external pH paper (yellow to red, rather than purple to
orange). Anhydrous Et2O was added to the point of turbidity, and as
soon as crystallization had actually started, more Et2O was added with
stirring, for a net total of 200 mL. After a couple of h standing,
the fine white crystalline 3,4-methylenedioxy-N-methoxyamphetamine
hydrochloride (MDMEO) was removed by filtration, Et2O washed, and air
dried to constant weight. There was obtained 1.7 g of a product with
a mp of 143-146 °C. The proton NMR was excellent with the N-methoxyl
group a sharp singlet at 4.06 ppm. Anal. (C11H16ClNO3) N.
DOSAGE: greater than 180 mgs.
DURATION: unknown
EXTENSIONS AND COMMENTARY: Why the interest in the N-methoxy analogue
of MDA? There are several reasons. One, this is an isostere of MDE
and it would be interesting to see if it might serve as a primer to
the promotion of the effectiveness of other drugs (see primer
discussion under MDPR). In one experiment, wherein a 60 microgram
dosage of LSD was used an hour and a half after a 180 milligram load
of MDMEO, there was no augmentation of effects. Thus, it would appear
not to be a primer. Another reason for interest was that the
material, although having an extremely similar overall structure to
most of the active MD-series compounds, is very much a weaker base.
And MDOH, which is also a very much weaker base than MDA, still shows
the action and potency of MDA. And, as this compound appears to be
inactive, base strength is not a sole predictor of activity.
The ultimate reason for making MDMEO was, of course, that it could be
made. That reason is totally sufficient all by itself.