by Erowid
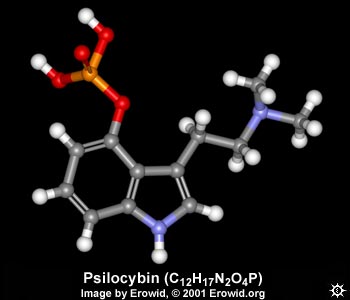
| NAME : | Psilocybin |
| CHEMICAL NAME : | 3-[2-(Dimethylamino)ethyl]-1H-indol-4-ol dihydrogen phosphate ester |
| ALTERNATE CHEMICAL NAMES : | O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine; Indocybin |
| CHEMICAL FORMULA | C12H17N2O4P |
| MOLECULAR WEIGHT | 284.25 |
| MELTING POINT | 220-228° C (Crystals from boiling water) |
| MELTING POINT | 185-195° C (Crystals from methanol) |
| pH | 5.2 (in 50% aq ethanol) |
| LD50 | 285 mg/kg i.v.(mice) |
| LD50 | 280 mg/kg i.v. (rats) |
| LD50 | 12.5 mg/kg i.v.(rabbits) |
| From the Merck Index 12th Edition | |
|---|---|
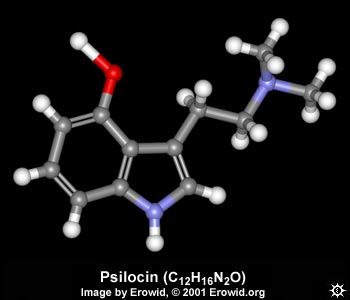
| NAME : | Psilocin |
| CHEMICAL NAME : | 3-[2-(Dimethylamino)ethyl]-1H-indol-4-ol |
| ALTERNATE CHEMICAL NAMES : | 4-hydroxy-N,N-dimethyltryptamine; psilocyn |
| CHEMICAL FORMULA | C12H16N2O |
| MOLECULAR WEIGHT | 204.27 |
| MELTING POINT | 173-176° C (plates from methanol) |
| From the Merck Index 12th Edition | |
|---|---|
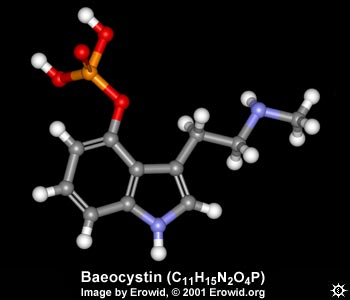
| NAME : | Baeocystin |
| CHEMICAL NAME : | 3-[2-(methylamino)ethyl]-1H-indol-4-ol dihydrogen phosphate ester |
| ALTERNATE CHEMICAL NAMES : | desmethyl psilocybine; 4-phosphoryloxy-N-methyltryptamine; baeocystin |
| CHEMICAL FORMULA | C11H15N2O4P |
| MOLECULAR WEIGHT | 270.28 |
| MELTING POINT | 254-258° C (crystals from methanol) |
| From Pharmacotheon | |
|---|---|
Brief Baeocystin Info
Psilocybin Extraction Notes
MERCK INDEX ENTRIES
8111. Psilocybin. 3-[2-(Dimethylamino)ethyl]-1H-indol-4-ol; O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine; Indocybin. C12H17N2O2P; mol wt 284.25. C 50.71%, H 6.03%, N 9.86%, O 22.51%, P 10.90%. The major of two hallucinogenic compounds in Teonanacatl, the sacred mushroom of Mexico, the other component being psilocin, q.v. from the fruiting bodies of Psilocybe mexicana Heim, Agaricaceae: Hofmann et al., Experientia 14, 107 (1958); Heim et al., Helv. Chim. Acto 42, 1557 (1959); Heim et al., Ger pat. 1,087,321 (1960 to Sandoz). Structure: Hofmann et al., Experientia 14, 397 (1958). Synthesis: Hofmann, Troxler, U.S. pat. 3,075,992 (1963 to Sandoz). Crystal structure: H.P. Weber, T.J. Petcher, J. Chem. Soc., Perkin Trans. II 1974, 942. Converted to psilocin in vivo. Toxicity data: E. Usdin, D.H. Efron, Psychotropic Drugs and Related Compounds (National Institute of Mental Health, Rockville, Md., 2nd ed., 1972) p 138. Reviews: Hofmann, Proc. 1st Int. Congr. Neuro-Pharm., Rome 1958, 446; Cerletti, Deut. Med Wochenschr. 84, 2317 (1959); Hofmann, Bull. Narcotics 23, 3 (1971).
Crystals from Boiling water, mp 220-228°; from boiling methanol, mp 185-195°. uv max (methanol): 220, 267, 290 nm (log E 4.6, 3.8, 3.6). pH 5.2 in 50% aq ethanol. Sol in 20 parts boiling water, 120 parts boiling methanol; difficultly sol. in ethanol. Practically insol in chloroform, benzene. LD50 in mice, rats, rabbits (mg/kg): 285, 280, 12.5 i.v. (Usdin, Efron).
Note: This is a controlled substance (hallucinogen) listed in the U.S. Code of Federal Regulations, Title 21 Part 1308.11(1995).
THERAP CAT: Psychomimetic
8110. Psilocin. 3-[2-(Diumethylamino)ethyl]-1H-indol-4-ol; 4-hydroxy-N,N-dimethyltryptamine; psilocyn. C12H16N2O; mol wt 204.27. C 70.56%, H 7.89%, N 13.71% O 7.83%. The minor hallucinogenic component of Teonanacatl, the sacred mushroom of Mexico. Isolated in trace amounts from the fruiting bodies of Psilocybe mexicana Heim, Agaricaceae: Hofmann et al., Experientia 14, 107 (1948); Heim et al., Helv. Chim. Acta 42, 1557 (1959). Prepn: Heim et al., Ger. pat 1,087,321 (1960 to Sandoz). Synthetic precursor of psilocybin: Hofmann, Troxler, U.S. pat. 3,075,992 (1963 to Sandoz). Psilocin, the 4-hydroxy analog of psilocybin, is formed by metabolic dephosphoylation of psilocybin and is the active species in thh central nervous system: Hoita, Weber, Toxicol Appl. Pharmacol. 4, 730 (1962). Crystal structure: T.J. Petcher, H.P. Weber, 730 (1962). Crystal structure: T.J. Petcher, H.P. Weber, J. Chem Soc. Perkin Trans. II 1974, 946. Review Hofmann, Bull. Narcotics 23, 3 (1971)
Plates from methanol, mp 173-176°. Amphoteric substance. Unstable in soln, esp. akaline soln. Very slightly sol in water. uv max: 222, 260, 267, 283, 293nm (log E 4.6, 3.7, 3.8, 3.7, 3.6).
Note: This is a controlled substance (hallucinogen) listed in the U.S. Code of Federal Regulations, Title 21 Part 1308.11(1995).

