Bulletin on Narcotics
Issue 1, 1971; 3-14
by Albert Hofmann
Teonanácatl, the "sacred mushroom" of the Aztecs
Ololiuqui
A comparative survey of Mexican hallucinogenic drugs
The use of the Mexican hallucinogens in experimental and practical psychiatry
The paramedical use of the hallucinogens
The country of origin of the majority and most important of the so-called magic, i.e. hallucinogenic drugs, is Central America. Magic drugs were already of great importance in the old Indian cultures of Mexico. The Spanish chroniclers and naturalists who came to the country soon after the conquest of Mexico by Cortez mentioned in their writings a great number of plants with intoxicating, stimulating, or narcotic effects; these plants were unknown in the Old World and were used by the Indians both in their medical practices and in their religious ceremonies. The cultic use and divine worship given to many of these drugs met with the disapproval of the Christian missionaries, who attempted by any means possible to liberate the Indians from this devilry. They were, however, only partially successful in this respect. The native population secretly continued using the drugs considered by them as holy even after having been converted to Christianity.
Three magic drugs were used mainly by the Aztecs and neighbouring tribes in their religious ceremonies and medical practices, which were strongly influenced by magical concepts; these drugs are still used today for the same purpose by the witch doctors in remote districts of Mexico. They are: 1. peyotl, a cactus species; 2. teonanácatl, certain foliate mushrooms; 3. ololiuqui, the seeds of bindweeds.
The first of these magic drugs to be analyzed was peyotl, the cactus Anhalonium Lewinii, this being done at the turn of the century. The alkaloid mescaline was found to be the psychoactive principle of peyotl. These investigations are to be considered as the first scientific studies in the field of psychotomimetics, and the two pioneer researchers who carried them out, Louis Lewin and Arthur Heffter, deserve a place of honour in the history of psychotomimetic research.
* Deputy Director, Sandoz A. G., Basle
Teonanácatl, the "sacred mushroom" of the Aztecs
About 60 years passed after the elucidation of "peyotl" before the second magic Mexican drug "teonanácatl" could be analysed in the Research Laboratories Sandoz Basle (Switzerland).
Teonanácatl, an Aztec word which could be translated as "sacred mushroom" or "God's flesh", was mentioned by the Spanish chroniclers as early as the sixteenth century, as were peyotl and ololiuqui. The most important source of information on this drug is de Sahagun's famous chronicle, "Historia General de las Cosas de Nueva Espańa", written in the years 1529-1590. It contains data on the use of intoxicating sacred mushrooms which were eaten by the Indians of Mexico at their feasts and religious ceremonies. From the Sahagun's chronicle and from other reports it can be seen that teonanácatl was not only ingested at social and festival occasions but also by witch doctors and soothsayers. The mushroom god-which the Christian missionaries called the devil- endowed them with clairvoyant properties, which enabled them, besides other things, to identify the causes of diseases and indicate the way in which they could be treated.
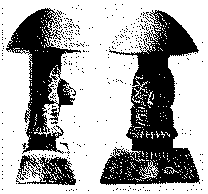
The use and the worship of these mushrooms by the Indians of Central America must be very ancient. In Guatemala so-called "mushroom stones" have been found. These are stones carved in the form of a pileate mushroom, in the stem of which the head or entire figure of a god is depicted (fig. 1). The oldest specimens found are over three thousand years old. It can therefore be concluded that the mushroom cult of the Indians dates back to more than thousands of years before Christ. More detailed historical data can be found in the monograph of the Wassons' "Mushroom, Russia and History" ([1] ).
Although this mushroom cult is very old, our knowledge of it is very recent. For some centuries the reports in the old chronicles were given surprisingly little attention, probably because they were regarded as extravagances of a superstitious age.
However, between 1936 and 1938, American investigators, i.e. Weitlaner, Reko, Johnson ([2] ), and Schultes ([3] , [4] ) ascertained that mushrooms are currently still being eaten for magical purposes by the natives in certain districts of southern Mexico.
FIGURE 1
Mushroom Stone ( Rietb rg Museum, Zürich)
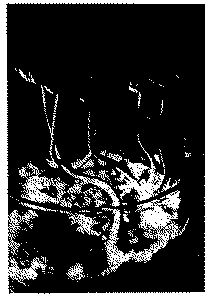
Systematic studies of the mushroom cult in its present form were later made by the amateur investigators R. Gordon Wasson and his wife, Valentina Pavlovna Wasson ([1] ). Between 1953 and 1955 they made several expeditions to the remote mountainous districts of southern Mexico to study the current use of the magic mushrooms. In the summer of 1955, R. G. Wasson was for the first time able to take active part in a secret nocturnal mushroom ceremony in Huautla de Jimenez, Province of Oaxaca, and probably was the first white man to ingest the holy mushrooms. This experience, which impressed him profoundly and which convinced him of the hallucinogenic effect of the mushrooms, has been described by him in detail in his monograph. On a further expedition in 1956 Wasson was accompanied by the mycologist Roger Heim, director of the laboratoire de cryptogamie du Muséum national d'histoire naturelle in Paris. Heim succeeded in identifying and classifying the most important types of mushrooms used for magical purposes by the Indians. These were foliate mushrooms (agaricales) of the family Strophariaceae, mostly new types, the greater part belonging to the genus Psilocybe, as well as one species of the genus Stropharia and one species of the genus Conocybe ([5] ). Subsequently cultures of some of these species were successfully grown in the laboratory (fig. 2). Artificial cultivation provided a very good yield, especially of one of these sacred mushrooms, namely, Psilocybe mexicana Helm. The chemical analysis of this mushroom material was effected in the Pharmaceutical Chemical Research Laboratories of Sandoz Ltd. in Basle by A. Hofmann and collaborators ([6] ). The mushroom extracts were first tested on animals. Studies were made of pupillary reaction and of piloerection in mice and of general behaviour in dogs. The results were not clear-cut and led to disagreement in the evaluation of the various extract fractions. After most of the very rare and valuable material (or rather the extract) had been given to the animals without effect, there was some doubt as to whether the mushrooms cultivated and dried in Paris were still active. A personal trial by the author of this article settled this fundamental point. He ate thirty-two dried specimens of Psilocybe mexicana weighing 2.4 g, a medium dose by Indian standards. The mushrooms exerted a marked psychotomimetic effect which was described as follows ([7] ):
FIGURE 2
Psilocybe mexicana Heim Laboratory culture (Photo: A. Brack)
"Thirty minutes after taking the mushrooms the exterior world began to undergo a strange transformation. Everything assumed a Mexican character. As I was perfectly well aware that my knowledge of the Mexican origin of the mushroom would lead me to imagine only Mexican scenery, I tried deliberately to look on my environment as I knew it normally. But all voluntary efforts to look at things in their customary forms and colours proved ineffective. Whether my eyes were closed or open I saw only Mexican motifs and colours. When the doctor supervising the experiment bent over me to check my blood pressure, he was transformed into an Aztec priest and I would not have been astonished if he had drawn an obsidian knife. In spite of the seriousness of the situation, it amused me to see how the Germanic face of my colleague had acquired a purely Indian expression. At the peak of the intoxication, about 1˝ hours after ingestion of the mushrooms, the rush of interior pictures, mostly abstract motifs rapidly changing in shape and colour, reached such an alarming degree that I feared that I would be torn into this whirlpool of form and colour and would dissolve. After about six hours the dream came to an end. Subjectively, I had no idea how long this condition had lasted. I felt my return to everyday reality to be a happy return from a strange, fantastic but quite really experienced world into an old and familiar home."
This personal study showed that the negative results of the tests on animals were due not to the mushroom material but to the animals used and that human beings provide a more sensitive index of substances with psychic effects than do animals.
With the aid of this reliable test on human beings it was possible to extract the active principles from the mushroom and to purify and crystallize them (fig. 3).
FIGURE 3
Psilocybin Psilocin
The main active component was named psilocybin, and an accompanying alkaloid, was named psilocin.
The dried mushrooms contain 0.2-0.4 per cent of psilocybin. Psilocin is present in trace amounts only. Elucidation of their structures led to the interesting result that these were novel indole derivatives. Degradation studies showed psilocybin to be 4-phosphoryloxy-N, N-dimethyltryptamine. Hydrolysis of psilocybin gave equi-molecular amounts of phosphoric acid and psilocin, which is 4-hydroxy-N, N-dimethyltryptamine (see diagram 1).
Diagram 1
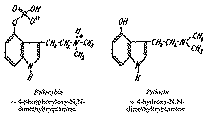
Psilocybin is a stable compound which is readily soluble in water and which is obtained in colourless crystals. Psilocin on the other hand is very sensitive to oxidation and difficultly soluble in water. Psilocybin is the first and only hitherto known natural indole compound which has a phosphoric acid radical. Psilocybin and psilocin are also novel in that they are substituted in the 4-position of the indole structure by a hydroxy radical.
These structures were confirmed by synthesis which was carried out by the pathway indicated in the following diagram 2.
Diagram 2
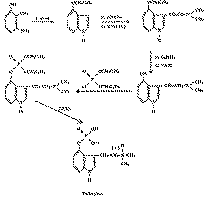
The synthetic production of psilocybin is much more rational than obtaining it from the mushrooms.
Later other Psilocybe species belonging to the teonanácatl group were also shown to contain psilocybin, usually together with a small amount of psilocin, e.g ., P. caerulescens Murr. var Mazatecorum Heim, P. Zapotecorum Heim, P. Aztecorum Heim, P. semperviva Heim et Cailleux, Stropharia cubensis Earle ([8] ). Stropharia cubensis is found not only in Central America but also in Cambodia and Thailand. Furthermore, psilocybin and psilocin were also found in North American Psilocybe species which are not known to be used for magical purposes, namely, in P. pelliculosa A. H. Smith, ([9] ) P. Cyanescens Wakefield, P. baeocystis Singer and Smith, and in the botanically closely related species Conocybe cyanopus (Atk.) Kuehner ([10] ).
The availability of the principles of the hallucinogenic mushrooms in the form of pure chemical compounds made it possible to study their pharmacological properties and mental effects.
Mental Effects of Psilocybin
Psilocybin and psilocin produce psychotomimetic effects in man which are similar to those produced by mescaline or LSD-25. The medium oral dose for man is 4 to 8 mg; it elicits the same symptoms as the consumption of about 2 g of dried Psilocybe mexicana fungus ([11] , [12] ).
The first analysis of the effects of psilocybin in man was made at the Psychiatric Clinic of the University of Basle and was based on personal studies made by several members of the staff of the Sandoz Research Laboratories ([13] , [14] ). More detailed investigations have been carried out by Delay and his associates in Paris ([15] , [16] ). As a result of these and many further investigations, of which only a few are mentioned in this article ([17] , [18] , [19] , [20] , [21] , [22] , [23] ), the effects of psilocybin can be described as follows: Oral doses of a few milligrams lead, after 20 to 30 minutes, to changes in the psychic sphere. The psychic symptoms produced by small doses, i.e., up to 4 mg, comprise effects on mood and environmental contact in that there frequently is a subjectively pleasant sensation of intellectual and bodily relaxation and detachment from the environment. Not infrequently these effects are associated with a pleasant feeling of physical tiredness and heaviness, but sometimes they are accompanied by a feeling of extraordinary lightness, a bodily hovering. With higher doses, 6 to 20 mg, more profound psychic changes are prominent and are associated with alterations in spatial and temporal perception and with changes in the awareness of the self and body image. Visual hypersensitivity is present and may lead to illusions and hallucinations. In this dreamlike state long-forgotten memories, even some from early childhood, are often recalled.
The psychotomimetic effects of psilocybin, psilocin, LSD, and mescaline were compared by Wolbach et al. ([24] ) and found to be qualitatively similar. The time course of the psilocybin and psilocin reactions is shorter than that of LSD or mescaline reactions. Psilocin is approximately 1.4 times as potent as psilocybin. This ratio is the same as that of the molecular weights of the two drugs. The development of "cross" tolerance between LSD and psilocybin supports the idea that these two drugs cause psychic disturbances by acting on some common mechanisms, or on mechanisms acting through a common final pathway ([25] , [26] , [27] ).
The influence of expectations and mood on responses to psilocybin was studied. It was found that positive expectations, in general, lead to positive experiences; anxiety or preoccupation leads to unpleasant experiences. Mood before the session is the best predictor of mood during the session: unpleasant depressed, anxious moods are intensified on the whole; pleasant moods lead to pleasant and varied experiences. Mystical or religious experiences are often reported after respective expectations and especially when these expectations are combined with high doses. A hypothesis was proposed, according to which the effect of psilocybin is to suspend or deactivate temporarily the cognitive-perceptual screening structures ([28] ).
The influence of psilocybin on the expressive capabilities for drawing were examined by Roubi ček and Drvota ([29] ) and by Volmat and Robert ([30] ).
Pharmacological Properties of Psilocybin
The first study of the effects of psilocybin on the whole animal and on isolated organs was carried out in the Pharmacological Department of Sandoz Ltd. in Basle under the direction of Dr. A. Cerletti.
Psilocybin does not exhibit typical effects on isolated organs (intestine, uterus, heart), with the exception of a pronounced inhibiting effect towards serotonin. On the entire animal, however, it has characteristic autonomic effects, namely, dilatation of the pupils, contraction of the nictitating membrane, piloerection, temperature increase, etc. This is an ergotropic excitation syndrome, which mainly results from a central stimulation of sympathetic structures ([31] , [32] ). In the electroencephalogram an activation may be detected which is characterized by a practically complete disappearance of the slow waves. The electroencephalographic "arousal" reaction of rabbits after psilocybin is not determined by a stimulating effect of the compound on the formatio reticularis but by an inhibition of thalamic substrates ([33] , [34] ). As opposed to this autonomic excitation syndrome produced by the central nervous system, the motor behaviour of the animals in general is strangely rather damped, which, however, does not exclude the simultaneous existence of a certain overexcitation towards outer stimuli.
A very characteristic effect of psilocybin is the regular enhancement of monosynaptic spinal reflexes, e.g., the patellar reflex of cats ([35] ).
The pharmacological effects of psilocin amply correspond to those of psilocybin qualitatively and quantitatively in studies performed thus far ([36] ). The phosphoric acid radical, therefore, does not appear to contribute to the pharmacological activity possessed by psilocybin, but as psilocybin is more stable towards chemical influences than psilocin, especially towards oxidation, the phosphoric acid radical could act biologically as a protective group.
The toxicity of psilocybin in animals is very low in comparison to the effective dose in man. The LD 50 for the mouse is 280 mg/kg; i.e., psilocybin is 2.5 times less toxic than mescaline in this test, while it has a 50 times higher psychotomimetic effect in man ([37] ).
Ololiuqui
Ololiuqui is the Aztec name for the seeds of certain convolvulaceous plants which have been used since prehispanic times by the Aztecs and related tribes, just as the sacred mushrooms and the cactus peyotl have been used in their religious ceremonies for magic and religious purposes. Ololiuqui is still used in our day by certain tribes, such as the Zapotecs, Chinantecs, Mazatecs, and Mixtecs, who live in the remote mountains of southern Mexico in comparative isolation, little or not at all influenced by Christianity.
An excellent review of the historical, botanical, and ethnological aspects of ololiuqui was given in 1941 by Schultes in his monograph "A Contribution to Our Knowledge of Rivea corymbosa: The Narcotic Ololiuqui of the Aztecs" ([38] ). The following information on the history of ololiuqui, its botanical identification and its past and present use have been taken mainly from Schultes' monograph.
One of the first descriptions and the first illustration of ololiuqui were given by Francisco Hernandez, a Spanish physician who between 1570 and 1575 carried out extensive research on the flora and fauna of Mexico for Philip II. In his famous "Rerum medicarum Novae Hispaniae thesaurus, seu plantarum, animalium, mineralium mexicanorum historia", which appeared in 1651 in Rome, Hernandez described and classified ololiuqui under the heading "De Oliliuhqui, seu planta orbicularium foliorum".
An extract of a free translation of the 1651 Latin version reads as follows: "Oliliuhqui, which some call coaxihuitl, or snake-plant, is a twinning herb with thin, green, cordate leaves, slender, green terete stems, and long white flowers. The seed is round and very like coriander."
In this work Hernandez claims that priests ate ololiuqui which induced a delirious state during which they were able to receive messages from the supernatural and communicate with their gods. He reported that priests saw visions and went into a state of terrifying hallucinations under the influence of the drug.
If we are to judge from the many ancient writers quoted in Schultes' monograph, ololiuqui must have been very extensively used in the valleys of Mexico in prehispanic times. It seems to have been more important in divinity than peyotl or teonanácatl. However, the medicinal use was also very extensive. Ololiuqui served to cure flatulence, to remedy venereal troubles, to deaden pain, and to remove tumours. Ololiuqui was believed to possess a deity of its own, which worked miracles if properly propitiated.
In spite of the above relatively good description and characteristic illustration by Hernandez, the botanical identification of ololiuqui caused a great number of discussions in professional circles. Finally, in 1897, M. Urbina identified ololiuqui as Rivea corymbosa Hall. f. (syn . Ipomoea sidaefolia (HBK)). This identification was confirmed by Schultes.
In Mexico, Rivea corymbosa is known and has been known by a number of different vernacular names, the more important of which are: Aztec: oliliuhqui, ololiuqui, coaxihuitl, cuexpalli; Chinantec: a-mu-kia, huan-mei, huan-men-ha-sei; Maya: xtabentum; Mazatec: no-so-le-na; Mixtec: yucu-yaha; Zapotec: bador, badoh, bitoo, kwan-la-si, kwan-do-a; Spanish: flor de la Virgen, la seńorita, manto, pascua, piule, semilla de la Virgen, yerba de las serpientes, yerba de la Virgen.
Ololiuqui was used by the ancient Aztecs not only as a potion but also as an ingredient of magical ointments. At the present time the crushed seeds are taken in water or in alcoholic beverages such as pulque, mescal, or aguardiente. Reko described in detail the present use of ololiuqui in his monograph "Magische Gifte" ([39] ). Usually the professional soothsayers, "piuleros", give their clients advice under the influence of the piule drink, another name for ololiuqui. Sometimes they also give the ololiuqui drank to their client or patient, who then replies to the piulero's leading questions in a narcotic-hypnotic state produced by the drug and thus reveals facts or discovers his illness, for which the piulero then finds the medicines.
The only report on chemical investigations with the seeds of Rivea corymbosa mentioned in Schultes' review on ololiuqui is that of the pharmacologist Santesson in Stockholm in 1937. He was, however, unsuccessful in isolating definite crystalline compounds. Alcoholic extracts produced a kind of narcosis or partial narcosis in frogs and mice. Certain chemical reactions seemed to suggest the presence of a gluco-alkaloid.
FIGURE 4
Seeds of Rivea corymbosa Hall. f. (left) and of Ipomoea violacea L. (right)
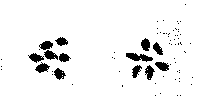
In 1955, the Canadian psychiatrist Osmond conducted a series of experiments on himself. After taking 60 to 100 Rivea seeds he passed into a state of apathy and listlessness accompanied by increased visual sensitivity. After about 4 hours, there followed a period in which he had a relaxed feeling of well-being that lasted for some longer time ([40] ). In contrast to these results, Kinross-Wright in 1958 published experiments performed on eight male volunteers who had taken doses of up to 125 seeds without any ascertainable effect in a single case ([41] ).
FIGURE 5
Rivea corymbosa Hall.f.

After the chemical investigations of the sacred Mexican mushrooms had come to a successful end, the writer decided also to tackle the problem of the third most important Mexican magic drug after peyotl and teonanacatl, namely, ololiuqui. Through the help of R. G. Wasson he was able to obtain authentic ololiuqui. He received two different samples of ololiuqui seeds, collected by a Zapotec Indian near Oaxaca in southern Mexico (fig. 4). One sample consisted of brown seeds, which proved on botanical classification to be Rivea corymbosa. The second sample, black seeds, was identical with Ipomoea violacea L. (syn. Ipomoea tricolor CAV.). These black seeds, called "badoh negro ", are used, especially in the region of the Zapotecs, in conjunction with, or instead of, "Badoh ", the brown seeds of Rivea corymbosa ([42] ).
Figure 5 shows the flowering plants which have been grown from these seeds.
The chemical investigations in the Sandoz laboratories led to the surprising result that the psychotomimetic principle of ololiuqui are ergot alkaloids ([43] , [44] , [45] ). "Badoh" and "Badoh negro" contain an alkaloid mixture of nearly the same composition (table I).
FIGURE 5
Ipomoea violacea L.

TABLE I Alkaloids of the seeds of Rivea corymbosa (L.) Hall. f. and Ipomoea violacea L. (In percentages)
| Alkaloids | Rivea corymbosa (ololiuqui, badoh) | Ipomoea violacea (badoh negro) |
|---|---|---|
|
d-Lysergic acid amide (ergine) |
0.0065 | 0.035 |
|
d-Isolysergic acid amide (isoergine) |
0.0020 | 0.005 |
|
Chanoclavine |
0.0005 | 0.005 |
|
Elymoclavine |
0.0005 | 0.005 |
|
Lysergol |
0.0005 |
— |
|
Ergometrine |
— |
0.005 |
|
Total alkaloid content |
0.012 | 0.06 |
The main component in both seeds is d-lysergic acid amide also called ergine. In later investigations it was found that ergine and isoergine were present in the seeds to some extent in the form of their condensation product with acetaldehyde, i.e. d-lysergic acid hydroxyethylamide and d-isolysergic acid hydroxyethylamide resp. The latter compounds are very easily hydrolyzed in the course of the extraction process to provide ergine resp. isoergine and acetaldehyde. Lysergic acid amide and isolysergic acid amide together with the corresponding hydroxyethylamides were found earlier also in ergot of Paspalumgrass ([46] ). Chanoclavine has previously been discovered in ergot of the tropical millet cob Pennisetum typhoideum Rich ([47] ). Elymoclavine was first isolated from ergot of the wild grass Elymus mollis Trin ([48] ). Lysergol was produced synthetically by reduction of d-lysergic acid ([49] ) before it was discovered to occur in nature as one of the active principles of ololiuqui. Ergometrine (ergonovine) is the alkaloid which is mainly responsible for the ultero-tonic hemostatic action of the ergot drug. It can also be obtained synthetically ([50] ).
Diagram 3
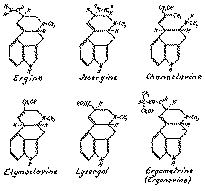
The structural formulae of the ololiuqui alkaloids are depicted in diagram 3.
The discovery of ergot alkaloids in ololiuqui i.e. in R. corymbosa and I. violacea was quite unexpected and of particular interest from the phytochemical point of view because lysergic acid alkaloids, which had hitherto been isolated only from the lower fungi of the genus Claviceps, Penicillium, or Rhizopus, were now, for the first time, found in higher plants, in the phanerogamic family Convolvulaceae. Subsequent chemical investigations in various other laboratories confirmed the occurrence of ergot alkaloids in lpomoea species ([51] , [52] , [53] , [54] , [55] , [56] ).
Pharmacological and Mental Effects
D-Lysergic acid amide (LA-111 = designation of the experimental drug) was tested pharmacologically during the course of investigations on d-lysergic acid diethylamide (LSD-25) and related compounds long before it was known to be a component of ololiuqui.
LA-111 elicited strong autonomic symptoms in rabbits, e.g. mydriasis, piloerection, and hyperthermia, which were accompanied by a general motor restlessness. The antiserotonin activity tested on the isolated rat uterus, has a value of 4 on a scale on which LSD-25 is 100 ([57] ).
Psychotomimetic activity of LA-111, having a marked narcotic component, could be ascertained from the first self-experiment by Hofmann ([45] ).
This action of d-lysergic acid amide was later confirmed by comparative systematic investigations by Solms ([58] , [59] ). He describes the action as follows: LA-111 induces indifference, a decrease in psychomotor activity, the feeling of sinking into nothingness and a desire to sleep... until finally an increased clouding of consciousness does produce sleep.
Only little information is available on the activityof d-isolysergic acid amide (isoergine). After taking 2.0 mg orally Hofmann experienced tiredness, apathy, a feeling of mental emptiness and of the unreality and complete meaninglessness of the outside world ([45] ).
D-Lysergic acid N-(1-hydroxyethyl) amide induces contractions in the isolated uterus of the guinea pig and in the rabbit uterus in situ, showing about 30-50% of the activity of ergometrine. In mice and rabbits it produced the syndrome of central sympathetic stimulation, such as piloerection, mydriasis, and hyperthermia, which suggests that it could have an LSD-like activity, but this hypothesis has not yet been verified by experiments on humans ([60] ).
Elymoclavine and lysergol elicit an excitation syndrome in various animals that is caused by a central stimulation of the sympathetic nerves ([61] ) which seems to indicate psychotomimetic activity. Results of clinical tests are not as yet available.
Psychotomimetic effects are unknown for ergometrine, which is used to a large extent in obstetrics as a uterotonic and hemostatic agent. In small dosages, which are administered for this purpose, the alkaloid apparently has no action on the psychic functions. Its occurrence in the alkaloid mixture of ololiuqui can thus have no significant effects on its mental action.
Furthermore, chanoclavine, which has no outstanding pharmacological activity, appears to play no part in the occurrence of the psychic effects of ololiuqui.
According to the results of experiments performed thus far with pure alkaloids, it appears that d-lysergic acid amide, d-lysergic acid N-(1-hydroxyethyl) amide, elymoclavine, and lysergol, and possibly also d-isolysergic acid amide are mainly responsible for the psychic effect of ololiuqui.
A comparative survey of Mexican hallucinogenic drugs
As mentioned at the beginning of this article, the major hallucinogenic plants and fungi originate from Mexico. These are the peyotl cactus, from which the first hallucinogen, mescaline, was isolated, Psilocybe mexicana Heim, the sacred mushroom containing psilocybin, and ololiuqui, a plant of the family Convolvulaceae, whose seeds have lysergic acid amides as their main active principles. The occurrence of lysergic acid amides in ololiuqui means that the synthetic compound lysergic acid diethylamide (LSD) ([62] ) has also to be included in the group of magic drugs of Mexico. LSD differs from the main constituent of ololiuqui only in that the two hydrogen atoms of the natural substance are replaced by two ethyl radicals. However, LSD is related to these sacred Mexican drugs not only in chemical structure, but also in its psychotomimetic properties. Subjects receiving mescaline daily develop tolerance to mescaline and cross-tolerance to LSD. Since persons directly tolerant to LSD are cross-tolerant to psilocybin ([26] ), it seems likely that persons directly tolerant to psilocybin would be cross-tolerant to mescaline. From this it has been concluded that mescaline, LSD, and psilocybin probably share common mechanisms of action or some common final pathway ([63] ).
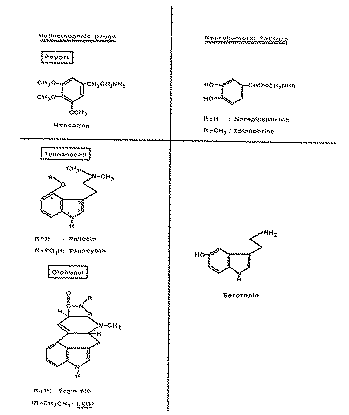
Hence to this extent the chemical structures of the Mexican hallucinogens form a common entity, and they are of particular interest, since they are related to endogenous active substances. This will be evident from the structural formulae shown below (diagram 4).
Mescaline, being a phenylethylamine derivative is structurally related to the neurohormonal transmitter substances norepinephrine and epinephrine. Psilocybin and psilocin as well as lysergic acid amide and the other ololiuqui constituents and LSD are indole derivatives, or more precisely tryptamine derivatives, like the neurohumoral factor serotonin (5 hydroxy-tryptamine). Because of this relationship between the Mexican hallucinogens and the neurohormones, serotonin and norepinephrine, it is probable that a mutual influence on the biochemistry of the central nervous system causes the psychotomimetic activity. Investigation of the relationships and interactions between endogenous neurohormonal factors and the hallucinogens is a rewarding facet of psychopharmacological research.
The use of the Mexican hallucinogens in experimental and practical psychiatry
Although the hallucinogens had long been known in the form of the ancient magic plants and fungi, it was not until their active principles had been isolated and prepared as pure chemical compounds, that they were amenable to scientific study and systematic use in medicine. The most thoroughly investigated hallucinogens are mescaline, LSD, which may be regarded as an improved modification of the active principle of ololiuqui, and psilocybin.
Whereas the hallucinogens have for some time been generally recognised to be valuable tools in experimental neurology and psychiatry, especially for the production of "model psychoses" ([64] ) which permit the study of the biochemical and electrophysiological processes involved in mental disorders, their use in psychotherapy is still controversial. The following effects of the hallucinogens are chiefly of value when applied as drug aids to psychoanalysis and psychotherapy:
-
They are able to release the patient from his autistic fixation and isolation by shattering and transforming his customary setting. As a result, the patient can obtain a more satisfactory relationship with the therapist.
-
Following the general psychic activation elicited by these drugs the resistance of the ego disappears, and forgotten or repressed memories may be evoked. Even experiences of very early childhood are often remembered. This is of major importance for the success of psychotherapy, particularly when the experiences concerned are those which have led to psychic trauma.
This kind of loosening of the psyche under the influence of psychotomimetics, followed by abreaction, which often takes place in a dramatic manner, has been named "psycholysis" by Leuner and Holfeld ([65] ). Some clinicians believe that the psycholytic experience deepens the self-understanding and insight of the patient, thus facilitating psychotherapeutic treatment, which can often be shortened by the use of psychotomimetic drugs.
Diagram 4
The main reason for failure with psychotomimetics and their subsequent rejection lies probably in the unskilled use of these agents. Drugs with such a profound psychic effect are not harmless when used indiscriminately and without due preparation of the patient. The psycholytic method of treatment requires zealous psychotherapeutic care of the patient, during and after administration of the drug. Special techniques have been developed for the wise and effective incorporation of the psychodelic experience in general psychotherapeutic treatment. It will doubtless be some time before the correct indications and optimum conditions are discovered for the therapeutic use of psychomimetics.
The paramedical use of the hallucinogens
An important aspect of psychotomimetic agents, which lies beyond the scope of natural science, is their paramedical use. Already pertaining to this is the use of magic drugs such as teonanŕcatl and ololiuqui for cultic and divinatory purposes which dates back from time immemorial and which is still found in various cultures. The use of psychotomimetics as "consciousness-expanding ", "mind-altering" drugs by nonmedical circles in our Western hemisphere today, which is also based on the scarcely describable, profound, almost magical psychic effects of these compounds, is also related in a certain sense to their cultic use.
There are fluent transitions from the strictly scientific medical-biological tests with these compounds, their use in psychological studies as educational aids, as tools for learning more about the potentialities of the human mind, and in studies on the nature of religious and mystical experiences, to their misuse as substitutes for intoxicants and for "kicks" in teenager and hippy circles. As an example of a study on the nature of mystical consciousness with the aid of a psychedelic drug the investigation of Pahnke ( [66] ) may be mentioned. He analysed the effect of psilocybin on students of theology during a Good Friday service of worship, with respect to the enhancement of the religious experience, and this enabled him to determine reactions that correspond to the criteria of a real mystical experience.
Aside from authors like Aldous Huxley who approved or even recommended chemical psychostimulants such as LSD, psilocybin and mescaline as modern forms of cultic drugs and as admissible aids in the human striving for transcendency, there are a number of others who consider such use of psychotomimetics an aberration. This opinion is expressed, for example, by Zaehner of Oxford in his book "Mysticism, Sacred and Profane" ([67] ).
Under the influence of psychotomimetic agents the sensitivity of our senses and the experience of sensory perceptions, which determine our conception of the universe, as well as the experience of our own personality, are changed. What corresponds to the reality : the everyday impression often perceived by dulled senses, or a picture of the world perceived by stimulated nerves and brain centres, sometimes experienced in ecstasy ? It is precisely because of the overwhelming impression of reality of these pictures and the accompanying feeling of unity with the universe that many persons believe the extraordinary condition elicited by these compounds to be a true mystical experience. On the other hand the opinion that, by the ingestion of a chemical substance, it may be possible to attain new knowledge or even insight of a religious nature in an easy way without moral or mental effort is difficult to reconcile with traditional views.
The controversy concerning the moral value or damage of the psychodelic experience and the question of whether a mental state induced by drugs is equal to a true mystical or religious experience will certainly continue for a long time to come. From the numerous tests so far carried out it may clearly be seen that the type and depth of the drug experience depend to a decisive extent on the mental make-up of the test person, on his expectations with regard to the experiment, and on the environment in which the psychedelic experience takes place. Mental and moral preparation is also of major importance in the cognitive qualities of the experience. Therefore, the result is in no way gratuitous but is the fruit of the mental structure and moral effort of the test person. The hallucinogen can only release and activate what is already present in the person taking it. All those who hope to progress without effort or work the easy way should be well aware of this.
Unlike the true narcotics of the opium and heroin class, etc., whose injurious effects develop in a chronic fashion, the hallucinogens do not give rise to addiction. Much is made of this by the proponents of the release of the hallucinogens for general use to support the innocuous nature of these substances. However, the hallucinogens are not less dangerous than the drugs of addiction, they are dangerous in a different way. The great danger associated with the use of hallucinogens without medical supervision is the possible occurrence of a "bad trip ", i.e. the development of severe confusional and anxiety states which may lead to suicide, or in psychically labile or youthful persons whose character is still not mature, permanent psychic traumata. The power of the Mexican hallucinogens to completely transform perceptivity, and the dangers inherent in such a process, readily explain why the primitive peoples imposed a taboo on these plants and fungi. For these peoples they were sacred and they were reserved for the medicine man for use in religious, ceremonial situations. Since in our society taboos no longer exist and the general trend is to throw aside the last inhibitions, the authorities have no alternative but to impose strict controls, restricting the use of hallucinogens to scientific research and medical applications. Legal measures are not an ideal solution to the drug problem, but in the present situation and in our present-day society they are the only choice for the time being.
References
001V. P. Wasson and R. G. Wasson, Mushrooms, Russia and History, Vol. II, pp. 215-322, Pantheon, New York, 1957.
002
J. B. Johnson, Ethnol. Stud., Gothenburg, 9, (1939)
003
R. E. Schultes, The Identification of Teonanácatl, botan. Museum Leaflets, Harvard Univ., 7 ([3] ) (1931).
004
R. E. Schultes, Am. Anthropol., 42, 429 (1940).
005
R. Heim and R. G. Wasson, Les Champignons hallucinogčnes du Mexique, ed. du Muséum national d'histoire naturelle, Paris, 1958.
006
A. Hofmann, R. Heim, A. Brack, H. Kobel, A. Frey, H. Ott, Th. Petrzilka and F. Troxler, Helv. Chim. Acta 42, 1557 (1959).
007
A. Hofmann, Chimia (Aarau) 14, 309 (1960).
008
R. Heim and A. Hofmann, Compt. Rend., 247, 557 (1958).
009
V. E. Tyler, Jr., Lloydia, 24, 71 (1961).
010
R. G. Benedict, L. R. Brady, A. H. Smith and V. E. Tyler, Jr., Lloydia 25, 156 (1962).
011
A. Hofmann, Chimia (Aarau) 14, 309 (1960).
012
Reports of self-experiments with the mushroom and with psilocybin by R. Heim, A. Hofmann, A. Brack and R. Cailleux, Les Champignons hallucinogčnes du Mexique, ed. du Muséum national d'histoire naturelle, Paris, 1958, pp. 272-285.
013
F. Gnirss, Schweiz. Arch. Neurol. Neurochir. Psychiat., 84, 346 (1959).
014
W. Rümmele, Schweiz. Arch. Neurol. Neurochir. Psychiat., 84, 348 (1959).
015
J. Delay, P. Pichot, Th. Lemperičre and P. Nicolas-Charles, Compt. Rend., 247, 1235 (1958).
016
J. Delay, P. Pichot, Th. Lemperičre, P. Nicolas-Charles and A.-M. Quétin, Ann. Med.-Psychol., 117, 891 (1959).
017
S. Malitz, H. Esecover, B. Wilkens and P. H. Hoch, Compr. Psychiat. 1, 8 (1960).
018
W. Rümmele and F. Gnirss, Schweiz. Arch. Neurol. Neurochir. Psychiat. 87, 365 (1961).
019
H. Heimann, Psychiat. Neurol. (Basel), 141, 69 (1961).
020
L. E. Hollister, Arch. Intern. Pharmacodyn. Therap., 130, 42 (1961).
021
H. Heimann, Schweiz. Arch. Neurol. Neurochir. Psychiat. 89, 214 (1962).
022
T. Leary, G. H. Litwin, A. B. Metzner, and R. Metzner, J. Nervous Mental Disease, 137, 561 (1963).
023
L. E. Hollister and B. M. Sjoberg, Compr. Psychiat., 5, 170 (1964).
024
A. B. Wolbach, E. J. Miner and H. Isbell, Psychopharmacologia 3, 219 (1962).
025
H. Isbell, Psychopharmacologia 1, 29 (1959).
026
H. Isbell, A. B. Wolbach, A. Wikler and E. J. Miner, Psychopharmacologia 2, 147 (1961).
027
H. A. Abramson, A. Rolo, B. Sklarofsky and J. Stache, J. Psychol., 49, 151 (1960).
028
R. Metzner, G. Litwin and G. M. Weil, Psychedelic Rev., 5, 3 (1965).
029
J. Roubicek and St. Drvota, Csl. Psychiat., 56, 44 (1960)
030
R. Vô1mat and R. Robert, Aesculape 43, 27 (1960).
031
A. Cerletti, in Neuro-Psychopharmacology (P. B. Bradley, P. Deniker and C. Radouco-Thomas, Eds.), Elsevier, Amsterdam 1959, p. 291.
032
H. Weidmann, M. Taeschler and H. Konzett, Experientia 14, 378 (1958).
033
M. Monnier, Experientia 15, 321 (1959).
034
J. F. Brodey, W. G. Steiner and H. E. Himwich, J. Pharmacol. Exptl. Therap. 140, 8 (1963).
035
H. Weidmann and A. Cerletti, Helv. Physiol. Pharmacol. Acta 17, C46 (1959).
036
A. Cerletti, Deut. Med. Wochschr. 84, 2317 (1959).
037
A. Hofmann, Svensk Kem. Tidsk. 72, 723 (1960).
038
R. E. Schultes, "A Contribution to our Knowledge of Rivea Corymbosa: The Narcotic Ololiuqui of the Aztecs ", Botanical Museum, Harvard Univ., Cambridge, Mass., 1941.
039
V. A. Reko, "Magische Gifte, Rausch- und Betäubungsmittel der Neuen Welt ", Ferdinand Enke, Stuttgart 1936.
040
H. Osmond, J. Mental Sci., 101, 526 (1955).
0491/A>
V. J. Kinross-Wright, in Neuro-Psychopharmacology (P. B. Bradley, P. Deniker and C. Radouco-Thomas, Eds.), Elsevier, Amsterdam 1959, p. 453.
042
Th. MacDougall, Bol. Centro Invest. Antropol. Mexico No. 6 (1960).
043
A. Hofmann and H. Tscherter, Experientia 16, 414 (1960).
044
A. Hofmann, Planta Medica 9, 354 (1961).
045
A. Hofmann, Botan. Museum Leaflets, Harvard Univ. 20, 194 (1963).
046
F. Arcamone, C. Bonino, E. B. Chain, A. Ferretti, P. Pennella, A. Tonolo, and L. Vero, Nature 187, 238 (1960).
047
A. Hofmann, R. Brunner, H. Kobel and A. Brack, Helv. Chim. Acta 40, 1358 (1957).
048
M. Abe, T. Yamano, Y. Kozu, and M. Kusumoto, J. Agr. Chem. Soc. Japan, 29, 364 (1955).
049
A. Stoll, A. Hofmann and W. Schlientz, Helv. Chim. Acta 32, 1947 (1949).
050
A. Stoll and A. Hofmann, Helv. Chim. Acta 26, 944 (1943).
051
W. A. Taber and R. A. Heacock, Can. J. Microbiol. 8, 137 (1962).
052
W. A. Taber, L. C. Vining and R. A. Heacock, Phytochemistry 2, 65 (1963).
053
D. Gröger, Flora, 153, 373 (1963).
054
W. A. Taber, R. A. Heacock and M. E. Mahon, Phytochemistry 2, 99 (1963).
055
H. C. Beyermann, A. van de Linde and G. J. Henning, Chem. Weekblad 59, 508 (1963).
056
J. W. Hylin and D. P. Watson, Science 148, 499 (1965).
057
A. Cerletti, E. Schlager, F. Spitzer and M. Taeschler, Schweiz. Apoth. Ztg. 101, 210 (1963).
058
H. Solms, J. Clin. Exptl. Psychopath. Quart. Rev. Psychiat. Neurol., 17, 429 (1956).
059
H. Solms, Praxis 45, 746 (1956).
060
A. Glässer, Nature 189, 313 (1961).
061
T. Yui and Y. Takeo, Japan J. Pharmacol. 7, 157 (1958).
062
"Die Geschichte des LSD-25 ", Triangel Sandoz Z. Med. Wiss. 2, 117 (1955).
063
A. B. Wolbach, H. Isbell and E. J. Miner, Psychopharmacologia 3, 1 (1962).
064
H. Leuner, "Die experimentelle Psychose ", Springer, Berlin, 1962.
065
H. Leuner and H. Holfeld, Psychiat. Neurol. 143, 379 (1963).
066
W. N. Pahnke, Thesis, Harvard Univ. Cambridge, Mass., June 1963.
067
R. C. Zaehner, "Mysticism, Sacred and Profane ", Clarendon, Oxford, 1957.

