The Neuropharmacology of Hallucinogens
a brief introduction
v1 Feb 2004 (first draft June 2003)
edited & published by Erowid
Citation: BilZ0r. "Neuropharmacology of Hallucinogens : a brief introduction".
NOTE: This article was the seed for the updated and more accurate article Neuropharmacology of Hallucinogens : a technical overview. This article should no longer be used as a source.
There are many classes of hallucinogens containing hundreds of different chemicals, but the two classically studied and used are the indoles (e.g. LSD, psilocybin, DMT) and the phenethylamines (e.g. 2C-B, mescaline, DOI). Although the drugs in these two classes produce different subjective effects (as do drugs within the same class), it can be argued that there is a similar quality to their effects. Perhaps a unified theory on the mechanisms of hallucinogens can be formulated from studying both of their pharmacologies. "There seems to be a fairly clear consensus that the key site for hallucinogen action is the 5-HT2a receptor
subtype."
-- Nichols 2004
Hallucinogens do not act on the raphe nuclei
Serotonin (5-HT) was identified as a neurotransmitter in the mid-1960s, when several clusters of neurons projecting throughout the cortex from the raphe nuclei in the brainstem were discovered. Judging by the molecular similarity of the indole hallucinogens to 5-HT, the action of the prototypical hallucinogen LSD on the raphe nuclei was studied. It was shown that LSD applied directly to the somatodendritic region (the region of a neuron which includes the cell body and the dendrites) of the raphe neurons inhibited their spontaneous activity (Aghajanian 1972). It was then shown that systemically injected (into animals' bodies) mescaline and other phenethylamines also decreased the spontaneous firing of raphe neurons, but when the chemicals were applied directly to the somatodendritic region of these neurons, they had no effect (Haigler and Aghajanian 1973). This meant that the chemicals were altering the firing of the raphe neurons indirectly and not through direct inhibition of that type of brain cell. It was later shown that the raphe-firing suppression is mediated by 5-HT-1A autoreceptors, and that selective 5-HT1A agonists, such as the anxiolytic busiprone, have no hallucinogenic action. Hence, LSD's action at the 5-HT1A receptor and raphe neurons is not necessary for -- and may be inconsequential to -- its hallucinogenic action (Aghajanian 1995).Hallucinogens work via the 5-HT2A receptor
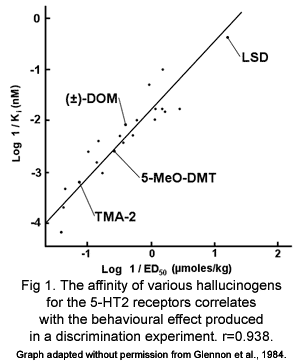
It was shown in the 1980s that the doses of both indole and phenethylamine hallucinogens that produce the same effects in mice and the doses taken recreationally by humans are mostly proportional to the affinity of that drug for the 5-HT2 receptors but not other 5-HT receptors (fig. 1) (Glennon 1984). This indicates that 5-HT2 receptors mediate the hallucinogenic effects of both classes of hallucinogens. In fact, it has been shown that the 5-HT2A/C receptors are the only receptors that phenethylamine and indole hallucinogens share affinity for (Aghajanian and Marek 1999).
Although there is a large body of evidence that indicates that the behavioural effects of hallucinogens are mediated by the 5-HT2A receptor, there is some research that points to the 5-HT2C receptor also mediating the effects. Rats trained to discriminate LSD or DOI from saline lose some of their accuracy when the hallucinogen is given in combination with a 5-HT2C antagonist (Smith 2003). This is not wholly surprising given hallucinogens' high affinity for the 5-HT2C receptor (Egan 2003) and its wide distribution in the CNS (Clemmet 2000). Whether the 5-HT2C receptor mediates any of the hallucinogens' truly psychedelic effects is yet to be seen. It seems unlikely, as the tolerance to hallucinogens that occurs with chronic use develops because of down-regulation of 5-HT2A receptors and not 5-HT2C receptors (Smith 1999). The 5-HT2A receptor, which now seems to be the only shared and clinically important target of hallucinogens, has been found throughout the cortex, and particularly in cortical pyramidal cells (Willins 1997).Hallucinogens are partial agonists at the 5-HT2A receptor
It was debated for many years whether the hallucinogens were agonists or antagonists at the 5-HT receptors. This question could not be answered until the various sub-types of receptors were distinguished. It has now been shown that LSD, DOI and some other hallucinogens are partial agonists at central 5-HT2A receptors, that is to say, they activate the receptors, but not to the same extent as serotonin itself (Marek andAghajanian 1996).| A comparison of human doses of selected hallucinogens with their potency using drug discrimination tests in LSD-trained rats | ||||||
|---|---|---|---|---|---|---|
| Drug | Ki 5-HT-2A (nM) | Ki 5-HT-2C (nM) |
Drug discrimination ED50 (µM/kg) | Potency relative to LSD (rat drug discrimination) | Human dose (mg) | Potency relative to LSD (human) |
| EthLAD | — | — | 0.02 | 185 | 0.04-0.15 | 140 |
| AllyLAD | — | — | 0.013 | 285 | 0.08-0.16 | 110 |
| LSD | 2-4 | 3-6 | 0.037 | 100 | 0.06-0.20 | 100 |
| ProLAD | — | — | 0.037 | 100 | 0.10-0.20 | 90 |
| DOB | 0.6 | 1.3 | 1.06 | 2.3 | 1-3 | 7 |
| DOI | 0.7 | 2.4 | 0.28 | 9.2 | 1.5-3 | 6 |
| DOM | 19 | — | 0.89 | 3.3 | 3-10 | 2 |
| Psilocin | 15-25 | 10 | 1.0 | 2.6 | 10-15 | 1 |
| DMCPA | — | — | 0.66 | 4.5 | 15-20 | 0.7 |
| MEM | 73 | 124 | 12 | 0.2 | 20-50 | 0.4 |
| MMDA-2 | — | — | 7 | 0.4 | 25-50 | 0.4 |
| Mescaline | 550 | 300 | 34 | 0.08 | 200-400 | 0.04 |
| Table from Nichols 2004 | ||||||
Hallucinogens share a common action at the locus coeruleus
Another brain stem nucleus affected by hallucinogens is the locus coeruleus (LC), a group of noradrenergic neuronal cell bodies which project throughout the entire central nervous system and receive a wealth of diverse sensory inputs. The LC shows spontaneous rhythmic activity and rapid firing upon sensory stimulation. Both indole and phenethylamine hallucinogens slow the spontaneous activity but potentiate the stimulation-dependent activation (Aghajanian 1980). The slowing of spontaneous activity in the LC seems to be mediated by 5-HT2A receptor activation of GABAergic inhibition, while the increase in stimulation-evoked activity of the LC is blocked by NMDA glutamate receptors antagonists (Chiang and Aston-Jones 1993). It is unknown whether the action of hallucinogens on the LC is relevant to their primary mental effects, but it seems unlikely to be a major factor. They probably don't play a major role because NMDA antagonists (ketamine, PCP) block the increase in stimulation-evoked activity of the LC but still cause hallucinations. Also, drugs which mimic increased LC activity (methamphetamine) rarely, if ever, cause true hallucinations. It is possible, however, that the anxiety that can be produced by hallucinogens is in part mediated by their effect on the LC and the increased sensitivity and response to the sensory hallucinations ( Aghajanian and Marek 1999).5-HT2A receptors are found at both pre- and post-synaptic sites
Looking at where the 5-HT2A receptors exist in the brain may tell us more about how hallucinogens produce their novel effects. It has been shown that the 5-HT2A receptor is most densely localized on the proximal apical dendrites of the cortical (especially layer V) pyramidal cells. This is mirrored by the fact that this is the only location where directly applied serotonin excites cells (Jakab and Goldman-Rakic 1998). Although one might assume that this means that 5-HT2A receptors directly activate the pyramidal cells (i.e. independent of action potential), this appears not to be the case because drugs which stop action potentials (tetrodotoxin) block the 5-HT2A induced excitation. Unfortunately, the picture is complicated by the fact that, although the 5-HT2A induced excitation is tetrodotoxin-sensitive (i.e. dependent of action potential), there is no evidence that 5-HT2A receptor activation leads to more action potentials (Aghajanian and Marek 1997).
5-HT2A receptors were also localized to the pre-synaptic surface of cells that synapse with cortical pyramidal cells (Jakab and Goldman-Rakic 1998). In line with this, 5-HT2A receptor agonists have been shown to excite cells via a mechanism that is blocked by presynaptic inhibitors (µ-opioid receptor agonists and group II/III mGlu agonists) (Aghajanian and Marek 1999).
Hallucinogens potentiate asynchronous synaptic transmission
Increased activation of layer V pyramidal cells seems to be the important action of hallucinogens (as this is where 5-HT2A receptors are mostly localized), but the question remains, how are these cells activated? As mentioned above, if hallucinogens are added to the area surrounding a pyramidal cell, there is an increase in the excitatory potentials that the cell receives, but there is no evidence of any increased firing of cells that synapse with the pyramidal cells (Aghajanian and Marek 1997). Even though there is no evidence that increased action potentials lead to pyramidal cell activation, they are not activated when action potentials are blocked. This leads to the idea that hallucinogens, via the 5-HT2A receptor, potentiate asynchronous excitatory transmission. Classically, "synchronous" synaptic transmission involves the action potential invading the presynaptic terminal, causing a voltage-dependent influx of Ca2+ that leads to a large-scale mobilization of synaptic vesicles and transmitter release. "Asynchronous" transmission is often recorded as "synaptic noise" but actually involves the low-level transmitter release seen (although rarely) for up to a second after synchronous transmission. It is believed that asynchronous transmission is molecularly distinct from synchronous transmission, as synchronous transmission is ablated when Ca2+ is replaced with Sr2+, but asynchronous transmission is not (Goda and Stevens 1994). It has been shown that in a normal pyramidal cell synapse, asynchronous transmission happens only rarely (or is present at an unrecordable level) but during treatment with hallucinogens it becomes very common, and highly recordable. It has also been shown that the excitatory potentials cortical pyramidal cells receive when treated with hallucinogens are not blocked when Ca2+ is replaced with Sr2+. Presumably, presynaptic 5-HT2A receptors, via their linkage to phospholipase C and IP3, increase presynaptic Ca2+ in such a fashion as to potentiate asynchronous excitatory glutamate transmission (Aghajanian and Marek 1998).Closing Comments
Many of the early questions raised about the action of hallucinogens seem to have been answered. Hallucinogens, working through 5-HT2 receptors, lead to increased excitation in the brain, especially in the frontal and temporal cortex. Although this is an explanation of the cellular events produced by hallucinogens, it does not come close to explaining the plethora of subjective effects that are the hallmark of hallucinogen intoxication. Some researchers put forward the hypothesis that hallucinogens produce their effect by limiting the filtering effect of the thalamus (so-called sensorimotor gating) and hence allow a flood of sensory information to overwhelm the cortex. Although this explanation sounds intuitive, not only do the dissociative anaesthetics (PCP, ketamine) produce the same deficient gating of sensory information but so do methamphetamine and even schizophrenia (Vollenweider and Geyer 2001). Granted, there are some similarities between the effects of these drugs and some symptoms of mental illness, but anyone who has taken mescaline and LSD knows there is a similarity between these two classes of drug that is not shared with ketamine or methamphetamine. Ultimately, only when science fully understands how the brain is capable of producing cognition will we be able to understand how not only the phenethylamine and indole hallucinogens, but also how other psychoactive drugs affect the mind.References #
- Aghajanian GK, Haigler HJ, Bloom FE. Lysergic acid diethylamide and serotonin: direct actions on serotonin-containing neurons in rat brain. Life Sciences. 1972 ; 11(13): 615-22. [ Abstract ]
- Aghajanian GK. Mescaline and LSD facilitate the activation of locus coeruleus neurons by peripheral stimuli. Brain Research. 1980; 186(2):492-8. [ Search PubMed ]
- Aghajanian GK. Electrophysiology of serotonin receptor subtypes and signal transduction mechanisms. In Bloom FE, Kupfer DJ (eds). Psychopharmacology: The Fourth Generation of Progress. New York, Raven Press, pp 451-460
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997; 36(4-5):589-99. . [ Search PubMed ]
- Aghajanian GK, Marek GJ. Serotonin 5-HT2A receptors enhance asynchornous excitatory transmission in pyramidal cells (layer V) of prefrontal cortex. Society for Neuroscience Abstracts. 1998; 24:1366. [ Search PubMed ]
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. ĆNeuropsychopharmacology. 1999; 21(2 Suppl):16S-23S. [ Search PubMed ]
- Chiang C, Aston-Jones G. A 5-hydroxytryptamine2 agonist augments gamma-aminobutyric acid and excitatory amino acid inputs to noradrenergic locus coeruleus neurons. Neuroscience. 1993; 54(2):409-20. [ Search PubMed ]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000; 39(1): 123-32. [ Search PubMed ]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology (Berl). 1998 Apr; 136(4): 409-14. [ Search PubMed ]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sciences. 1984; 35(25):2505-11. [ Search PubMed ]
- Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proceedings of the National Academy of Sciences of the United States of America. 1994; 91(26):12942-6. [ Search PubMed ]
- Haigler HJ, Aghajanian GK. Mescaline and LSD: direct and indirect effects on serotonin-containing neurons in brain. European Journal of Pharmacology. 1973; 21(1): 53-60. [ Abstract ]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. . Proceedings of the National Academy of Sciences of the United States of America. 1998; 95(2):735-40. [ Search PubMed ]
- Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. Journal Pharmacology and Experimental Therapeutics. 1996; 278(3): 1373-82. [ Search PubMed ]
- Marek GJ, Aghajanian GK. Serotonergic amplification of aplically derived EPSPs in neocortical pyramidal cells. Society for Neuroscience Abstracts. 1998; 22:1323. [ Search PubMed ]
- Nichols DE. Hallucinogens. Pharmacology & Therapeutics 101 (2004) 131– 181, 2004; 101:131-181. [abstract]
- Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology. 1999; 144(3):248-54. [ Search PubMed ]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/-)DOI] in C57BL/6J mice. Psychopharmacology (Berl). 2003 Feb; 166(1): 61-8. [ Search PubMed ]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Research Bulletin. 2001 Nov 15; 56(5): 495-507. [ Search PubMed ]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997; 27(1): 79-82. [ Search PubMed ]