Synthesis of 2,5-Dimethoxyallylbenzeneby Psycho Chemist(4-methoxy-phenoxy)-allyl ether1 mole (124g) of 4-methoxyphenol is dissolved in 500 mL methanol, in which 1.25 moles sodium hydroxide (50g) are dissolved previously. With external cooling and stirring, 1.1 moles (121g) of allyl bromide (caution!) is added dropwise, the internal temperature is below 40°C. After the addition, the mixture is refluxed for 60 min. The mixture is poured on 1 kg ice, and extracted 5 times with methyl-tert.-butyl ether. The combined extracts are dried over magnesium sulphate (50 g), and evaporated. The residue is fairly pure (4-methoxyphenoxy)-allyl ether. 2-hydroxy-5-methoxy-allylbenzeneThe crude (4-methoxyphenoxy)-allyl ether is heated for 6 hrs. to reflux, so Claisen rearrangement takes place. The product is then cooled to room temperature and mixed with 500 mL 2 N sodium hydroxide solution, and extracted 2 times with petrol ether. The extracts are discarged, containing non-phenolic products. The aqueous layer is acidified with 37% hydrochloric acid and extracted 5 times with 200 mL methyl-tert.-butyl ether. The extracts are dried with 50 g magnesium sulphate, and evaporated to give fairly pure 2-hydroxy-5-methoxy-allylbenzene. 2,5-dimethoxyallylbenzeneThe crude 2-hydroxy-5-methoxy-allylbenzene is dissolved in 500 mL methanol + 50 g sodium hydroxide. 1 mole dimethyl sulphate is added dropwise with stirring , the internal temperature is below 40°C (cooling). After the addition, the mixture is refluxed for 3 hrs, and poured on 1 kg ice. The product is extracted with 5x200mL methyl-tert.-butyl ether, the extracts are dried over magnesium sulphate, and evaporated. The residue is steam-distilled, and the product is extracted with methyl-tert.-butyl ether, and the solvent is evaporated to give 55% over-all-yield 2,5-dimethoxyallylbenzene. The use of diethyl sulphate in the third step will yield 2-ethoxy-5-methoxyallylbenzene. 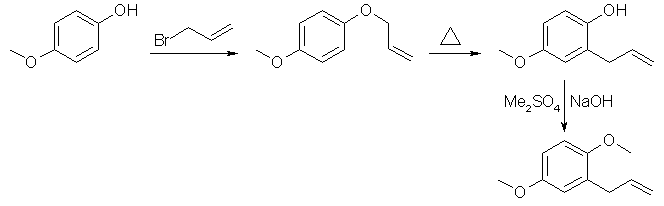 |