5-Methoxy-N-methyltryptamine and hordenine (p-hydroxyphenethyldimethylamine) have been isolated from reed canary grass (Phalaris arundinacea L.).
A report1 of the occurrence of " staggers " in sheep confined to pastures in which the perennial grass Phalaris tuberosa predominates led us to investigate the chemical constituents of a species of Phalaris (P. arundinacea L.) growing in the grounds of these laboratories. From this grass, which is free from any fungal parasite, 5-methoxy-N-methyltryptamine and hordenine were isolated.
Whilst hordenine has been found in the seedlings of cereals2 and in the Cactaceae, Anhalonium and Trichocereus2, 5-methoxy-N-methyltryptamine is apparently a new naturally-occurring alkaloid and is of interest biogenetically and pharmacologically because of its relation to 5-hydroxytryptamine.
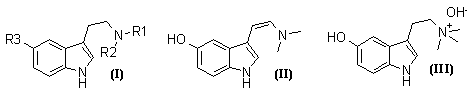
Of the series of tryptamines (I; R1=H or Me, R2=H or Me; R3=H or OH or OMe), all except (I; R1=R2=H, R3=OMe) and (I; R1=R2=Me, R3=OMe) occur naturally3,4, as do dehydrobufotenine (II) and bufotenidine (III)4.
The total alkaloids from P. arundinacea gave, with p-dimethylaminobenzaldehyde in 65% v/v sulfuric acid, a green solution which became blue on dilution with water (cf. tryptamine) and with the same reagent in ethanol containing hydrochloric acid, a deep blue, in contrast to gramine and tryptamine which gave pale pink and purple colours, respectively.
Paper chromatography of the alkaloids (Whatman No. 1 paper) with n-butanol-water-acetic acid (12:5:3), followed by spraying with p-dimethylaminobenzaldehyde in cyclohexane and treatment with hydrogen chloride, showed the presence of two indoles, a large blue spot (Rf = 0.58) and a smaller pink spot (Rf = 0.70).
The alkaloids were separated by chromatography on alumina, the course of the separation being followed by paper chromatography and colour reactions. The first runnings contained no indoles but from them hordenine was isolated. The later fractions, yielded a crystalline hydrochloride, C12H15ON.HCl (Rf = 0.58 in the above solvent mixture), containing no phenolic hydroxyl groups and giving a blue colour with sodium nitroprusside and acetone but not with acetaldehyde, characteristic of a secondary amine5, hence suggesting the structure methoxy-N-methyltryptamine. Comparison of the ultraviolet spectrum with that of tetrahydro-6-methoxycarbazole6 indicated that the methoxy-group was probably located in the 5-position. Conclusive proof was finally obtained by synthesis of 5-methoxy-N-methyltryptamine, the melting points of the hydrochlorides and the ultraviolet spectra being identical.
The second, minor, indole alkaloid (Rf 0.70) has not been identified; although concentrating in the mother liquors from which 5-methoxy-N-methyltryptamine is crystallised, it is present only in small amounts. It is not gramine but on the paper chromatogram it gives the same colour reaction and has the same Rf as 3-diethylaminomethylindole.
Experimental
Extraction
Minced grass (4.5 kg) was extracted at 60°C for 5 h with ethanol (45 L). After being kept overnight, the liquor was removed by decanting and pressing, and concentrated under reduced pressure to 2L; water (2 L) was added, and the mixture was heated at 60°C for 5 min. and filtered. The pale brown filtrate was saturated with sodium chloride, strongly basified with 20% aqueous sodium hydroxide, and extracted with chloroform (2x1.5 L). The combined chloroform extracts were dried (Na2SO4) and concentrated under reduced pressure.
The residue was dissolved in chloroform (10 mL) and chromatographed on alumina (4x20cm); elution was continued with chloroform and 5-mL fractions were collected. Each fraction was tested with: A: Millon's reagent, B: dimethylaminobenzaldehyde (0.125 g. in 65% sulfuric acid containing 0.1% v/v of 5% ferric chloride solution), C: addition of water to the mixture obtained with reagent B, and D: ferric chloride solution, with the following results:
| Fraction no. | Weight | A |
B |
C |
D |
| 4-11 | 1.0g |
Red |
Brown |
Green |
Blue-green |
| 12-60 | 1.2g |
Brown |
Green |
Blue |
- |
Fractions 4-11 were combined and concentrated under reduced pressure, the residue was dissolved in dry ether, and dry hydrogen chloride was passed through the solution. The precipitated hydrochloride was washed with ether and crystallised from alcohol-acetone giving platelets, mp 176-177°C (hordenine hydrochloride7, mp 176.5-177.5°C) from which the base was obtained as prisms, mp 116-117°C (hordenine,7 mp 117-118°C), from aqueous alcohol. It gave a methiodide, mp 228-229°C (prisms from ethanol) (hordenine methiodide,7 mp. 229-230°C). Fractions 12-60 were combined and concentrated under reduced pressure and the residue was converted in ether into the hydrochloride, which formed prisms (from ethanol-acetone), mp 165-166°C (mp not depressed on admixture with synthetic 5-methoxy-N-methyltryptamine hydrochloride). The picrate formed orange-red prisms, mp 220-221°C (decomp).
5-Methoxy-N-p-toluenesulfonyltryptamine
5-Methoxytryptamine8 (6 g) in benzene (72 mL) was shaken for 1 h with p-toluenesulfonyl chloride (7.2 g), potassium hydroxide (3.72 g,), and water (36 mL). After acidification of the solution with hydrochloric acid, the solid was filtered off, washed with water, dried (P2O5), and crystallised from benzene, giving plates, mp 123-124°C (8.9 g).
5-Methoxy-N-methyl-N-toluene-p-sulfonyltryptamine
Interaction of 5-methoxy-N-p-toluenesulfonyltryptamine (6g), ethanol (10 mL), 80% aqueous sodium hydroxide (3.6g), and methyl iodide (3.4g) during 12 h at room temperature gave prisms (5.5g) (from ethanol), mp 118-119°C.
5-Methoxy-N-methyLtryptamine
The p-toluenesulfonyl derivative (5g) was dissolved in liquid ammonia (200 mL) and pellets of sodium were added until the liquid remained blue for some ten min. The colour was discharged by addition of ammonium chloride, the ammonia was evaporated, and the residue dissolved in water (10 mL). The solution was basified with sodium hydroxide solution and extracted with ether. The ether solution was dried (Na2SO4) and the residue distilled at 150°C/0.05 mmHg (bath temp) to give a pale yellow oil (3 g). Dry hydrogen chloride was passed through its ethereal solution and the precipitate was washed with ether and crystallised from ethanol-acetone, giving prisms, mp 166-167°C.
References
- 8th Annual Report of the Commonwealth Scientific and Industrial Research Organisation, 1956.
- Manske and Holmes, "The Alkaloids". III. p. 320. New York, 1949,
- White. New Zealand Sci. Technol.. 1944, 25, B. 137; Yarashevski, J. Gen. Chem. (U.S.S.R.), 1939. 9, 595; 1940, 10. 3781; 1941. 11, 157; Hochstein and Paradies, J. Amer. Chem. Soc., 1957, 79, 5735; Erspamer. Rend. Sci. Farm., 1, 1954; Jaques and Schachter, Brit. J. Pharmacol., 1954, 9, 53; Adams and Weiss. Nature. 1956, 178, 421; Bowden, Brown. and Batty, ibid., 1954, 174, 925; Collier and Chesher, Brit. J. Pharmacol., 1956, 11, 186; Mathias. Ross, and Schachter, Nature, 1957, 180, 658.
- Wieland, Kons, and Mittasch, Annalen. 1934, 513, 1; Jensen and Chen, J. Biol. Chem., 1936, 116, 87; Erspamer, Pharmacol. Rev., 1954, 6, 427.
- Kharichkov, J. Russ. Phys. Chem. Soc.. 1906, 38, 1407.
- Chalmers, Openshaw. and Smith. JCS., 1957, 1115.
- Henry, "The Plant Alkaloids", 4th Ed., Churchill. London. 1949.
- Abramovitch and Shapiro. JCS. 1956. 4589.