MDA by Al/Hg reduction of MDP2P oxime in acidic EtOHby Antibody[ Back to the Chemistry Archive ] For obvious reasons an alternative catalyst to sodium was sought for the reduction of the oxime of MDP-2-P to its amine derivative. This investigation led to experimentaion with various solvent systems including THF, MeOH and EtOH both aqueous and non-aqueous, acidic and neutral, with various acids being employed. Hazards and low yield ruled out THF, and long reduction times and moderate yeilds led to the abandon of MeOH systems. Aqueous systems afforded superior yeilds with all solvents triied. Non acidic solvent-sytems failed altogather. HOAc provided higher yeilds than HCl. Different acid proprtions were triied with 10 equivalents producing the highest yeilds. The amount of Al used also impacted yeilds as well as the amount of MDOH by-product produced. With increases boosting overall yeild but lowering yeild of MDOH. 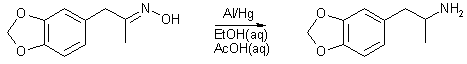
It should also be noted that the following solvent/redxn system was sucessfully employed in the manufacture of MMDA and DMMDA but in lower yeilds, 10% and 50% respectively. It failed repeatedly with DMMDA-2 altogather. Experimental In a 4l beaker with mag stirring, 0.75 moles of activated Al (19.5g) [1] is added to 1l 95% EtOH and 100mls dH2O, followed by 0.33 moles oxime of MDP-2-P (65g) [2],and 3 moles HOAc (180g). The Rxn is heated to 60C and heat removed. There follow three additions of 0.75 moles of activated Al at 30 minutes, 1 hour, and two hours. Temperature was maintained at 60C by placing beaker in a cold water bath as necessary. There is a vigourous evolution of hydrogen as the rxn progresses, care must be taken the rxn vessel does not overflow. An additional 150mls 95% EtOH and 15mls dH2O is added at 2hours. At 3 hours the rxn is a viscous gel which has stopped the stir bar. An additional 300mls 95% EtOH and 30mls dH2O is added . The rxn was allowed to stir until it has returned to room temperature, during which time 1 l of 15M NaOH was prepared and cooled. The rxn vessel was placed in a cold water bath and the basic solution was added slowly over 20 minutes, with care being taken the tempertaure did not rise above 60C. 500g of NaCl was added, much of which precipitated after stirring. 500mls toluene is added with stirring. The toluene/EtOH/amine layer is separated and decanted into 750mls of dH2O, causing the EtOH to migrate to the aqueous layer. The toluene layer is separated and the aqueous/alcoholic layer is extracted 2 times with 250mls toluene. The pooled toluene extracts are washed once with 400mls dH20 and once with 400mls brine, then dried through MgSO4 and gassed w/ dry HCl gas, furnishing 54g MDA HCl (0.267 moles, 81%) .
Addendum by Chromic: A variation on Sonson's oxime preparation: Mix:
Stir, then add:
Then do the 1.5hr reflux, cool in fridge, and suction filter a la Sonson. The calculations were a pain, so I thought I'd include them here so other people don't have to repeat them. |