Recent examples of the sustained interest in reagents for the conversion of carboxylic acids to chlorides, esters, amides and peptides are;
- 2,4,6-Trinitrofluorobenzene for the preparation of amides and esters2
- Isocyahides for peptide synthesis3
- Oxalyl chloride and catalytic DMF for the conversion of carboxylic acids to chlorides via the TBDMS esters4
- Esterification of carboxylic acids in the presence of DCC and a catalytic 4-dialkylaminopyridine5
Another useful regent for these purposes is cyanuric chloride (CC), readily available as an intermediate for the (manufacture of reactive dyes, fluorescent brightening agents and agricultural pesticides.
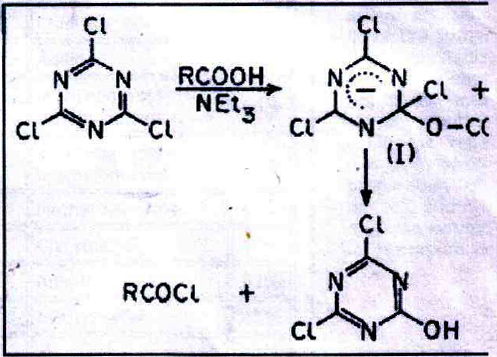
Senier recorded in 1886 the preparation of acetyl and benzoyl chlorides by heating the sodium salts with cyanuric chloride at 100?C for 8 hours6. Refluxing CC with a large excess of glacial acetic acid has been suggested as a method for the preparation of cyanuric acid, acetyl chloride being simultaneously formed. In the method now described the reaction is carried out at room temperature and CC separates as an insoluble product; the solution containing the acid chloride and any unconverted acid can be used directly for further reactions.
When CC in acetone is treated with 1 or 2 mol of carboxylic acid and 1-2 mols of triethylamine (TEA), the acid chloride is rapidly formed, presumably via the σ-adduct (I) resulting from a nucleophilic attack of RCOO- on CC. CC is converted into insoluble dichlorohydroxy- or chlorodihydroxy-s-triazine8, which have also been characterized as the corresponding dianilino or monoanilino derivatives9.
Experimental
General procedure
Add TEA (0.02mol) to a solution of the carboxylic acid (0.02mol or 0.01 mol of a dicarboxylic acid, etc.) and CC (0.01 mol) in acetone (20ml or minimum volume required for clear solution) at 20-30?C. After stirring for 3hrs when no CC remains in solution, acetone is remove under reduced pressure and the acid chloride taken up in carbon tetrachloride. Alternatively, when the desired product is an ester or amide, the alcohol, phenol or amine (0.02mol) is added to the reaction mixture, which is then stirred for 2hrs. The triazine derivative is filtered off and the acetone solution worked up as usual.
| Acid | RNH2/ArOH/ROH | Product10 | Yield |
| Acetic | Aniline | Anilide | 84% |
| p-Aminophenol | p-Hydroxyacetanilide | 55% |
|
| p-acetoxyacetanilide | 35% | ||
| Trifluoroacetic | Aniline | Anilide11 | 64% |
| L-Valine | N-Trifluoroacetyl- L-valine12 |
50% | |
| Oxalic | Aniline | Anilide | 52% |
| Malonic | - / Aniline | Chloride or Anilide | 0% |
| Succinic | Aniline | Anilide | 55% |
| Z-glycine | 2,4,5-Trichloro- phenol |
Trichlorophenyl ester13 |
41% |
| Z-glycine | Gly-OEt | Z-Gly-Gly-OEt14 | 40% |
| Z-L-valine | Gly-OEt | Z-L-Val -Gly-OEt15 | 45% |
| Boc-L-valine | Gly-OEt | Boc-L-Val-Gly-OEt16 | 38% |
| Phenylacetic | Aniline | Anilide | 86% |
| Benzoic | - | Chloride | 81% |
| Aniline | Anilide | 87% | |
| o-Phenylene- diamine | N,N-Dibenzoyl- o-phenylenediamine | 68% | |
| o-Aminophenol | N,O-Dibenzoyl- o-aminophenol | 73% | |
| Methanol | Methyl benzoate | 82% | |
| p-Nitrobenzoic | - | Chloride | 58% |
| Cinnamic | Aniline | Anilide | 93% |
| Aspirin | Methanol | Methyl ester | 45% |
| 3-Hydroxy-2-naphthoic | Aniline | Anilide17 | 61% |
Three dipeptides were prepared from (1) Z-glycine, (2) Z-L-valine and (3) boc-L-valine. After treatment with CC and TEA, glycine ethyl ester hydrochloride is added as a suspension in acetone (10 ml) and TEA (0.04 mol). The reaction mixture is added to ice-water and extracted with chloroform. The dipeptide, recovered from the chloroform solution after the removal of unconverted acid and amine, is then crystallized14-16. The three peptides and Z-glycine trichlorophenyl ester were characterized by their NMR and mass spectra in addition to their melting points.
The yields recorded in the Table are of the isolated products after purification by acid and base washing, when they were chromatographically homogenous and had mp's about 5?C lower than the literature values, but before the crystallization; the recovery uncovered acid was not taken into account. A simple routine procedure using the regenerated TEA was followed for the preparation of esters and amides, and no attempt was made to optimize conditions for maximum yields.