Cyanomethylation of Indole with Diethylaminoacetonitrile[ Back to the Chemistry Archive ]To these interesting examples of direct alkylation with tertiary amines should now be added the alkylation of indole with diethylaminoacetonitrile. This alkylation proceeds at ca. 170°C in the absence of a solvent to produce indole-3-acetonitrile in moderate (33-44%) yield. The nitrile was identified by comparison of its infrared spectrum with that of an authentic sample, by the melting point of its picrate and by hydrolysis to the acid in excellent yield. This identification incidentally indicates that the nitrile obtained by the "cyanomethylation" reaction was a very pure sample, and that the method here developed is a convenient one for the preparation of indole-3-acetonitrile on a small scale. Unfortunately attempts to scale up the preparation resulted in diminishing yields; this is probably due to the great sensitivity of the cyanomethylation reaction to temperature fluctuations. At temperatures below 160°C very little reaction ensues at all, while around 180°C tar formation supervenes. Diethylaminoacetonitrile [2,3]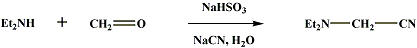
This preparation should be carried out under a good hood since poisonous hydrogen cyanide may be evolved. To a solution of 312 g. (3 moles) of sodium bisulfite in 750 ml. of water in a 3-l. beaker is added 225 ml. of a 37–40% formaldehyde solution, and the mixture is warmed to 60°. After cooling to 35°, 219 g. (309 ml., 3 moles) of diethylamine is added with hand stirring, and the mixture is allowed to stand for 2 hours. The beaker containing the reaction mixture is placed under a good hood, and to it is added a solution of 147 g. (3 moles) of sodium cyanide dissolved in 400 ml. of water with efficient stirring so that the two layers are thoroughly mixed. After 1.5 hours the upper nitrile layer is separated and dried over 25 g. of Drierite; it weighs 299–309 g. (90–92%). The crude product is purified by distillation; the portion boiling at 61–63°/14 mm., n25D 1.4230, amounts to 298–302g. (88–90%). Indole-3-acetonitrile [1]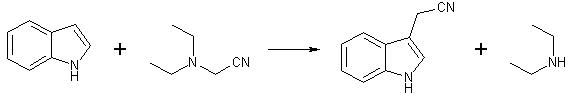
A mixture of 11.8 g. (0.1 mole) of indole and 22.4 g. (0.2 mole) of diethylaminoacetonitrile was maintained at an internal temperature close to 170°C under nitrogen for six hours in a flask equipped with a reflux condenser. The excess diethylaminoacetonitrile was then removed at water pump pressure and the residue was submitted to distillation at the oil pump. After a small forerun of indole, indoleacetonitrile was collected at 144-152°C (0.03 mm.) or 160-165°C (0.2 mm.) (lit. 157°C (0.2 mm.)) as a viscous, slightly cloudy liquid weighing 5.2-6.9g. (33-44%). The picrate melted at 128- 129° without recrystallization (lit. 127-128°C) and hydrolysis of the nitrile with 20% aqueous potassium hydroxide yielded the acid, m.p. 162-164°C (dec.) in 85% yield (lit. m.p. 164-165°C (dec.), yield 86%). We were unable to find a solvent for the reaction, aromatic hydrocarbons or aliphatic alcohols having been proved unsuitable. Attempts to scale up the preparation led to a drop in yield. References [1] JACS 3589 (1953)
|