Abstract
Ephedrine and pseudoephedrine, commonly used in clandestine laboratories within the United States to synthesize methamphetamine by means of reduction, are utilized within the Union of Soviet Socialist Republics (USSR) in synthetic oxidation with potassium permanganate to form 2-methylamino-1-phenylpropan-1-one. This ketone product of methamphetamine, termed "ephedrone" and "Jeff", is profiled with the use of spot tests, infrared spectrophotometry, mass spectrometry, gas chromatography, ultraviolet spectroscopy; and hydrogen (1H) and carbon-13 (13C) nuclear magnetic resonance spectroscopy.
Ephedrone, also referred to by its street name, "Jeff", is 2-methylamino-1-phenylpropan-1-one (see Fig. 1, No. 2). Herein considered for its forensic significance, this oxidation product of ephedrine has become a substantial drug of abuse in the Union of Soviet Socialist Republics (USSR), In addition, the reduction product of ephedrine, methamphetamine (Fig. 1, No. 3), also known as speed, crank, and ice, is a serious drug of abuse in the United States, A recent review by Allen and Cantrell has addressed the numerous synthetic reductions to methamphetamine1.
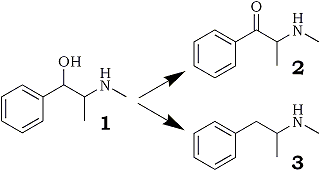
Fig 1.
Reaction pathways of ephedrine [1], which may be
reduced to methamphetamine [3] or oxidized, as
described in the present study, to ephedrone [2].
Ephedrone, although not reported as a street drug within the United States, has been responsible for numerous drug overdose deaths within the USSR, Jeff is known (as one knows in terms of rumors) to be clandestinely synthesized by potassium permanganate oxidation of ephedrine, although chromium trioxide appears as the oxidant in a U.S. patent held by Parke-Davis & Co2. Other literature references3-10 have reported construction of ephedrone by alternative syntheses without relying on oxidation from ephedrine. Our attempts to apply hearsay information regarding the clandestine permanganate synthesis, along with prudent chemical manipulations, resulted in the ketonic product and the information reproduced herein.
Chemicals and Equipment
All the chemicals used were analytical grade. The ephedrine hydrochloride and pseudoephedrine hydrochloride were obtained from Aldrich Chemical Co. (Milwaukee, Wisconsin), Gas-liquid chromatography (GLC) was performed on a Hewlett-Packard Model 5890 instrument (Palo Alto, California), Ther capillary column was a J&W DB-17 cross-linked methyl silicone column, 30 m by 0.53 mm inside diameter (ID), with a 1.0 µm film thickness. Helium was the carrier gas in the capillary column (with a linear velocity of 30 cm/s, and nitrogen-phosphorus detector (NPD) was used to generate the signal.
Infrared data were obtained using a Perkin-Elmer (Norwalk, Connecticut) Model 283 grating spectrophotometer and pressed potassium bromide (KBr) pellets, Nuclear magnetic resonance (NMR) data were obtained using a Varian (Palo Alto, California) Model Gemini-300 with deuterochloroform (CDCl3) as a solvent and tetramethylsilane (TMS) as a standard.
Mass spectral data were obtained using electron impact (EI) fragmentation at 70 eV on a Hewlett-Packard (Palo Alto, California) model 5970 mass selective detector. The sample inlet was through the gas chromatograph, which was fitted with a 12-m by 0.32-mm ID fused silica capillary column coated with cross-linked 5% phenylmethyl silicone HP-5 at a film thickness of 0.52 µm. The oven temperature program was as follows: initial temperature, 100°C; initial hold, 1 min; temperature program rate, 15°C/min; final temperature, 280°C; final hold, 3 min.
Experimental
2-Methylamino-1-Phenylpropan-1-One (Ephedrone, Jeff)
A 2000-mL Erlenmeyer flask, equipped with a magnetic stirring bar, was charged with methylene chloride (200 mL), acetic acid (10 mL), water (100 mL), potassium permanganate (2 g), and ephedrine hydrochloride (2 g). The solution was stirred at room temperature for 30 min. This was followed by the addition of sufficient sodium hydrogen sulfite to reduce the precipitated manganese dioxide, The aqueous phase was made basic with 5N sodium hydroxide (NaOH), and the methylene chloride was separated. The organic layer was extracted with 0.5N sulfuric acid (H2SO4). Isolation of the acid layer, followed by basification with sodium bicarbonate and extraction with methylene chloride (50 mL, three times), removed the product into the organic phase. The solvent was concentrated by rotary evaporation, followed by column chromatography through neutral alumina with methylene chloride. Solvent removal through rotary evaporation produced a colorless liquid which was dissolved in hexane. Gaseous hydrochloric acid was bubbled into the hexane to precipitate the amine hydrochloride to produce a 1 gram (50%) yield of 2-methylamino-1-phenylpropan-1-one hydrochloride.
Results and Discussion
Ephedrone, like methamphetamine, possesses one asymmetric center. Depending upon the synthetic precursor, l-ephedrine (1R,2S), or d-pseudoephedrine (1S,2R), the product expected would be d-ephedrone (2S) or l-ephedrone (2R), respectively. [Note by Rhodium: This stereochemistry is in error. While the configuration of l-ephedrine is written correctly, d-pseudoephedrine has the configuration (1S, 2S) and not the one given above. As oxidation of the alcohol eliminates the chiral center differing between the two, both will give rise to l-ephedrone and not the oxymoronic isomer specified above (d-ephedrone has R configuration, and is not produced by oxidation of the above precursor isomers).] However, depending upon the heat of the reaction or harsh extraction conditions the enolizable ketone will result in racemic dl-ephedrone.
Fig. 2a.
Spectral characterization of Ephedrone [2]:
IR spectrum.
Fig. 2b.
Spectral characterization of Ephedrone [2]:
(top) electron impact mass fragmentation,
(bottom) 300-MHz 1H-NMR spectrum.
The infrared data (see Fig. 2) substantiates the secondary amine hydrochloride moiety (2937, 2696, and 2461 cm-1), which is unchanged when ephedrine is changed to ephedrone. Furthermore, the absence of an amide carbonyl stretching eliminates the possibility of N-formyl oxidative formation, as is typical of some amine-permanganate oxidations, such as those for cocaine11 and other amines12,13. The carbonyl stretching frequency at 1700 cm-1 argues for the presence of a benzoyl ketone. This is confirmed splitting of the aromatic proton resonances in the hydrogen (1H) NMR, which is typical of an orthogonal carbonyl with the aromatic ring (see Fig. 2a). The carbon-13 (13C) NMR data show good correlation between the experimental and calculated chemical shifts14 (see Table 1a). At the same time, the mass spectral data confirm the nominal mass (C10H13NO = 163), and the amine driven beta bond cleavage is exhibited in the m/z 58 ion (see Fig. 2b, top spectrum).
Conclusion
The foregoing results confirm that the permanganate oxidation product of ephedrine is 2-methylamino-1-phenylpropan-1-one, referred to by its street names Ephedrone and Jeff. The spectral data have been included (see Fig. 2) for forensic usefulness.