Properties of Asarone and Anethole Epoxidesby Uemura[ Back to the Chemistry Archive ] IntroductionAt The Hive there is currently going on some research on the preparation of phenyl-2-propanones from propenylbenzenes via their corresponding epoxides. This small write-up gives some details on the physical properties of two epoxides, namely anethole epoxide (4-methoxyphenyl-1,2-epoxypropane) and asarone epoxide (2,4,5-trimethoxyphenyl-1,2-epoxypropane). The preparation is based on a reaction originally proposed by Osmium. Uemura modified the precedure to allow re-use of the MeCN used in this synthesis. A more OTC method for the epoxide synthesis has been published by Chromic. Epoxide Synthesis (described for anethole) [N1]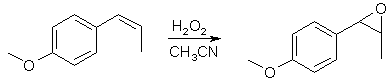
19.25g anethole (0.13 mol, ~20ml) is dissolved in 200ml of a 1:1 mixture of acetonitrile and methanol. 2g Na2CO3 are added and the mixture is stirred at room temperature with slow stirring [N2]. Within 10-15 minutes 14.75ml 0.13 mol) 30% H2O2 is added dropwise under stirring [N3]. Stirring is continued at room temp for 18-24 h. After the time has elapsed, the MeCN/MeOH solvent is distilled off on a waterbath. [N4] An oily water-epoxide suspension remains. The crude epoxide is extracted with ether or another non-polar solvent. The ether is washed with brine and dried over Na2SO4 [N5]. After drying the ether is distilled off on a water bath and the last traces of the ether are removed in vaccuum leaving the crude epoxide in the flask. Crude Anethole Epoxide: This is a clear yellow pleasant smelling oil, yield ~90%.
Purification of the epoxidesTo purify the crude epoxides, a slow vaccuum distillation is used. Anethole Epoxide Distillation: The yellow crude epoxide was distilled at 13mmHg and 15.4g (70%) of a water-clear, very pleasant smelling oil came over between 118-126°C. Höring reports 132°C/11 mmHg [N7]. In the distillation flask a deeper yellow oil with a higher boiling point remains [N8]. Asarone Epoxide Distillation: The golden-brown oil (17g) is distilled under maximum vacuum (2-4 mmHg). Within 125-135°C a clear light yellow oil distills over. The yellow oil has the typical epoxide smell, very similar to the anethole epoxide, but very different from the crude asarone epoxide. Yield 8.3g. In the distillation flask 4.3g of a red thick oil remains, which after cooling solidifies but does not crystallize [N9]. Pictures of the purified asarone epoxide and the high-boiling residue. DiscussionAs the 'law of difficulty conservation' predicted, the epoxidation of asarone is much more picky than the one of anethole. In case of anethole consistent results and clean products have been received. In case of asarone, the yield of the desired epoxide is low (8.3g from 20ml asarone, ~40%) and it seems to be essential to purify already the crude epoxide before going ahead [N10]. A first thermal re-arrangment run on anethole epoxide went smoothly [N11], the similar attempt for the asarone epoxide should be worth a try as soon as sufficient clean epoxide is available. Notes [1] Same procedure has been applied for asarone.
|