This technique combines the speed and separation of 'flash' chromatography with use of the cheaper TLC grade silica, simple operation and absence of special apparatus requirements. In 'dry flash' column chromatography, the silica column is eluted by suction instead of using top pressure, removing the risk of bursting glassware. Additionally the column is eluted by adding predetermined volumes of solvent and is run dry before addition of the next fraction.
These features make the procedure readily adaptable to gradient elution and this is in fact the preferred way of developing such columns. As its name suggests, gradient elution involves developing a column with progressively more polar combinations of eluting solvent. This can confer very real time advantages for the removal of polar compounds from a column in the latter stages of a separation, without losing the separation qualities of a relatively non-polar eluting system at the beginning for the less polar components.
Do not forget that this is the only instance when you should allow air to get into a chromatography column during development. In fact the whole procedure appears to fly in the face of all of the principles of classic chromatography. but it can give results at least as good as the standard flash technique at much reduced cost. It is particularly useful for the separation of enormous (in chromatographic terms at least!) quantities of material up to 50g — although such columns should not be attempted by the inexperienced. This technique has been the subject of a certain degree of quantification by one of the authors (LMH) but has been in fairly general use in various laboratories for a long time.
The equipment
The apparatus needed is simply that for filtration under reduced pressure using a cylindrical porosity 3 sinter funnel attached to a round-bottomed flask by means of a cone and socket adapter with a side arm for attachment to a water aspirator (Fig. 3.74). The amount of sample to be purified determines the size of sinter funnel to use and the volume of fractions to collect. Suggested guidelines are shown in Table 3.14.
Choosing the solvent system
The eluting solvent system used should be that in which the desired component has an Rf value of 0.5 by TLC analysis. Although no solvent is particularly disfavoured for this technique, various combinations of hexane, diethyl ether, ethyl acetate and methanol are adequate for the majority of separations. As the system is under reduced pressure, some of the solvent collected will evaporate and may cool the receiving vessel to such an extent that atmospheric moisture condenses on the apparatus. This does not affect the efficiency of the separation but, if the chromatography is prolonged, some water may find its way into the collected fractions. Use of the less volatile heptane instead of light petroleum helps somewhat in this respect.
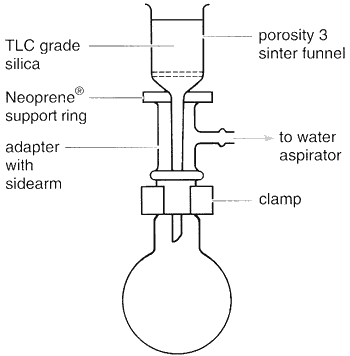
Fig. 3.74
Apparatus for 'dry flash' column chromatography.
Table 3.14
Guideline size and volume parameters
for 'dry flash' chromatography.
Sinter Diameter |
Weight of Silica |
Sample Weight |
Fraction Volume |
30 mm | 15 g | 15-500 mg | 10-15 mL |
40 mm | 30 g |
0.5-3 g | 15-30 mL |
70 mm | 100 g |
2-15 g | 20-50 mL |
Packing the column
The silica used for this type of chromatography is TLC grade silica without the gypsum binder. This is cheaper than the silica sold for use in flash chromatography which, in any case, is too free flowing for use in these dry columns. The weight of silica recommended for each size of funnel is sufficient to leaven head space at the top of the funnel for loading solvent when the column has been packed and compacted under suction. The silica may be weighed out as indicated in the table, but it is easier just to fill the funnel to the brim with lightly packed silica. Application of suction causes the silica to compact, leaving the head space for solvent addition. During this initial compaction there is a tendency, particularly with the larger sized columns, for the silicate shrink away from the sides of the funnel or to form cracks which may remain unseen in the body of the adsorbant. To ensure good packing, press down firmly on the surface with a glass stopper, particularly at the edges, using a grinding motion. Do not worry about the state of the adsorbant surface as this can be flattened off easily when finished by repeated gentle tapping around the sides of the funnel with a spatula.
When satisfied that the column has been thoroughly compacted, pre-elute the column with the least polar component of the elution system. If the packing has been carried out properly, the solvent front will be seen descending in a straight, horizontal line. Keep the silica surface covered with solvent during the pre-elution, until solvent passes into the receiving flask, and then allow the silica to be sucked dry. Remember to check the back as well as the front of the column for any irregularities. If a regular solvent front is not obtained, simply suck the column dry, recompact it and repeat the pre-elution procedure. There is no excuse for attempting a separation with an improperly packed column. Note that the surface of the compacted silica is relatively stable on addition of solvent and does not require any protective layer of sand.
Loading the sample and eluting the column
Dissolve your sample in the minimum possible volume of pre-elution solvent and apply it evenly to the surface of the silica with the column under suction. Rinse the sample container and add the washings to the column until all of the sample has been transferred. lf the sample does not dissolve easily in the pre-elution solvent, dissolve it in the least polar combination of the elution solvents in which it is readily soluble.
Commence gradient elution with the same solvent combination as was used to load the sample onto the column, following the guidelines in the table for the size of fraction to use (use the smaller volumes for more difficult separations ).Allow the column to be sucked dry and transfer the first fraction to a test tube or any other convenient receptacle, rinsing both the flask and the stem of the funnel. Whilst the column is being sucked dry, prepare the next fraction. increasing the quantity of the more polar component by about 5%. Repeat the elution procedure. Continue the gradient elution in this manner until eluting with the pure, more polar component alone. and then continue with this as necessary. It is often advantageous to interrupt the gradient elution temporarily when the desired component is eluting from the column and continue with the same solvent mixture for a few fractions.
The progress of the separation should be followed by TLC analysis of the fractions. However. as a rough guide, the desired product is usually eluted from the column when the gradient elution reaches that solvent mixture in which the material would have an Rf value of 0.5 on TLC. When quantities of material of more than about 100mg are purified. elution of product from the column is often indicated by frothing on the underside of the sinter. If the product is a solid. it may crystallize out in the stem of the funnel or the receiving flask, particularly with separations of larger quantities of material. Be sure to rinse thoroughly both the flask and the funnel stem between fractions, and check that the solid does not obstruct elution from the column.
The typically low degree of lateral diffusion of the product bands with this technique usually means that pure compounds elute in relatively few fractions. reducing the number of cross-contaminated fractions. The material recovery from the column should be excellent if the crude sample does not contain polymeric material.
Disposal of the silica
After the elution is complete, suck the silica dry and then transfer it to the silica residues bin. Generally a sharp tap with the funnel held upside down will cause the whole of the adsorbant to fall out as a single plug of material. As always, care should be taken not to produce large quantities of silica dust in the atmosphere of the laboratory.