Abstract
The isomerization of eugenol to isoeugenol was investigated by employing catalysis by KOH in amyl alcohol or glycerol, or by RhCl3. A number of factors which affect the reaction (solvent, temperature, molar ratios, presence of water) were examined.
Isoeugenol is widely used in the fragrance industry. Synthetically, it is obtained by the isomerization of eugenol: The reaction is carried out either by the so-called alkaline procedure1-6 (heating with KOH, most often in higher alcohols), or by the action of group VIII metals or their compounds serving as catalysts7-14. Both of these procedures have been studied in order to assess the effect of reaction parameters on the process of isomerization.
Experimental
Chemicals: eugenol, was a commercial sample (bp 122°C/1.6 kPa, density=0.5425g/ml. nD=1.5409 [20] Astrid Praha), potassium hydroxide, amyl alcohol and glycerol were analytical purity grade (Lachema Brno). Ethanol denaturated with methanol was technical grade, dried and distilled (United Distilleries, Prague). Rhodium(III)chloride was commercial (Safina, Vestec).
Apparatus and working procedure: The reactions were carried out in a 15 ml flask provided with a reflux condenser, stirrer and thermometer and placed in thermally insulated aluminium block with controlled temperature. In the case of alkaline isomerization, amyl alcohol or glycerol and potassium hydroxide were introduced into the reactor, and eugenol was added after reaching the reaction temperature. In the isomerization catalyzed by rhodium(III)chloride the reaction was started by introducing an ethanolic solution of rhodium(III)chloride into the thermostated eugenol. Samples of the reaction mixture taken during the reaction were adjusted (neutralization of the samples from the alkaline isomerization with dilute H2SO4) and analyzed by gas chromatography using a Chrom 5 apparatus with flame-ionization detection. A glass column (1.2m x 2.5mm) packed with 15% Carbowax 20M on Chromaton NAW-HMDS was used. The analyses were performed at 190°C.
Results And Discussion
Fig. 1.
Alkaline isomerization of eugenol in glycerol
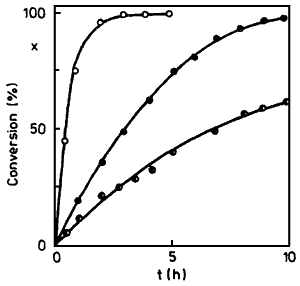
Temperature 150°C, molar ratio 1:9 between
Eugenol/Glycerol. The curves show reaction
rates using 7.3, 4.4 and 2.2 eq. KOH
Isomerization by KOH
The introductory experiments were carried out according to the literature2. The reaction mixture consisting of eugenol (10 mmol), potassium hydroxide (73 mmol) and amyl alcohol (67 mmol) was heated to 150°C for 10 h. The mixture was very viscous, and the samples solidified in cooling. In an effort to prevent this, the amount of potassium hydroxide was gradually reduced (to 4.4 or 2.2 mol/mol). As a result, the reaction was slowed down: with 2.2 mol/mol KOH the degree of conversion after 10 h was only 0.6, with 4.4 mol/mol KOH, it was 0.95.
In further experiments, amyl alcohol was replaced by glycerol which led to an improved consistency while at the same time enabling the reaction temperature to be raised. Typical reaction courses can be seen in Fig. 1.
From Fig. 1, a rise in the reaction rate with increasing amount of KOH can be seen. An increase in the reaction temperature to 173°C or 195°C (amount of KOH 4.4 mol/mol) brought about an increase in the reaction rate; the activation energy as determined from three points was 94.5 kJ/mol. In these cases too, however, the viscosity of the reaction mixture was quite high. With respect to difficulties in manipulation such procedure is not adequate for application.
Catalytic isomerization
The experiments are summarized in Table 1.
Table 1
Isomerization of eugenol with rhodium(III)chloride
| No. | Temp. | Eugenol (mmol) | RhCl3 (µmol) |
Alcohola (µmol) |
H2O (mmol) |
Initial reaction rate mmol*s-1*mL-1 |
Half time | Conversion |
Total reaction time |
| 1 | 95°C |
10 | 5.1 | 1.3 | - | 2.65 |
205 min |
55% |
7 h |
| 2 | 95°C |
10 | 5.1 | 1.3 | 0.42 | 2.65 |
205 min |
55% |
7 h |
| 3 | 95°C |
10 | 5.1 | 1.3 | 1.70 | 1.72 | 505 min | 48% |
7 h |
| 4 | 143°C |
10 | 5.1 | 1.3 | - | 13.20 | 16 min | 98% |
7 h |
| 5 | 143°C |
10 | 5.1 | 1.0 | - | 1.89 | 266 min | 66% |
7 h |
| 6 | 143°C |
10 | 10.3 | 1.3 | - | 25.30 | 7 min | 99% |
5 h |
| 7 | 143°C |
10+10 | 10.3 | 1.3 | - | 17.70 | 265 min | 55% |
5 h |
a. Ethanol was used in all cases, only in experiment 5 was isopropyl alcohol employed
It is known from the literature15-17 that rhodium(III)chloride used as the isomerization catalyst in this study appears in both hydrated and anhydrous form, and that samples of different provenance differ greatly from each other (in color, solubility, catalytic activity, etc.). All experiments were performed with anhydrous rhodium(III)chloride dissolved in ethanol or isopropyl alcohol.
The first three experiments in Table 1 demonstrate the negative effect of the addition of water to the reaction mixture. Moreover, the effect of the potentially present free hydrogen chloride (in an amount comparable with added water) was also tested. The result again was negative. Experiments 4 and 5 show that the replacement of ethanol by isopropyl alcohol leads to a considerable slowing down of the reaction. The activation energy of the reaction estimated from the reaction rates in experiments 1 and 4 was 42.6 kJ/mol. Experiment 7 was carried out so that after completion of the reaction in experiment 6, the same amount of eugenol was again added to the reaction mixture and the heating was continued.
It can be seen from a comparison that the activity of rhodium(III)chloride decreases considerably during the reaction. The reaction course remained unaffected if the experiments were carried out in nitrogen.
It may be inferred from the experiments that the best procedure for the isomerization of eugenol is the one in which the reaction is catalyzed by rhodium(III)chloride dissolved in ethanol, by rhodium(III)chloride in an amount of 1 mmol per 1 mol of the starting eugenol (order of magnitude), at about 140°C, when after about three hours a virtually total conversion is achieved.