Abstract
The direct conversion of halohydrins to ketones can be achieved by irradiation in benzene or toluene in the presence of small amounts of p-toluenesulfonic acid. A two step conversion of terminal alkenes to methylketones is thus achieved with good yields and inexpensive reagents.
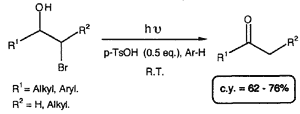
Few examples of the direct conversion of halohydrins to ketones have been reported. Rearrangement of their magnesium salts has led to the corresponding ketones under thermal conditions1. Oxidation using silver carbonate on Celite has given similar results2. More recently, methods have been reported which require catalytic amounts of Pd(OAc)2 combined with tri-o-tolylphosphine3 or which use cobalt(I) complexes in stoichiometric quantities4. We have found that under irradiation at 254 nm in benzene or toluene and in the presence of p-toluenesulfonic acid (0.5 eq.), direct conversion of bromohydrins to ketones was achieved.
Bromohydrins have been synthesised according to a published procedure from the corresponding alkene using NBS in aqueous DMSO5 or by reduction of the bromoketone with NaBH4 in methanol. Results of their irradiation carried out in our laboratory appear in the following table.
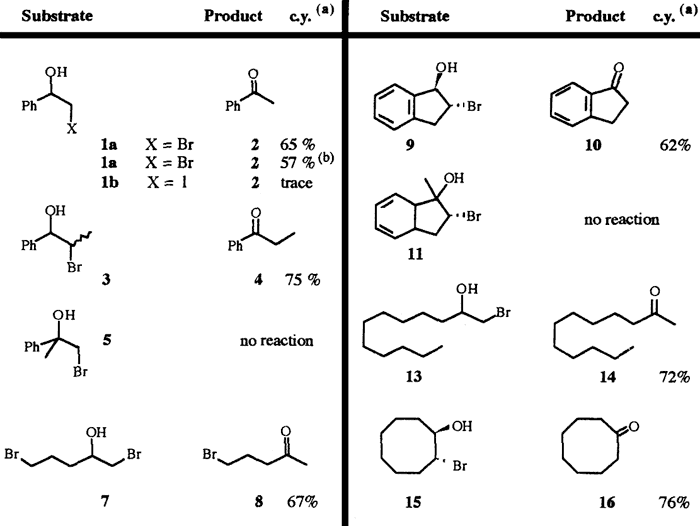
In general, bromohydrins lead to the corresponding carbonyl compounds in a few hours and in high yields. The presence of TsOH improves the yields slightly (irradiation of 1a). In contrast with the reaction involving the magnesium salts no rearrangement occurred when a bromohydrin bearing a tertiary alcohol (5 and 11) was submitted to irradiation. Moreover the presence of one bromide on the chain (7) is compatible with the reaction conditions. Otherwise, compared to bromohydrins, iodohydrins give only traces of the expected products, even after irradiation over a long time.
This procedure represents a convenient two step method to convert selectively a terminal alkene to the corresponding methylketone. Compared to previous published processes2-4, inexpensive reagents can be employed; thus we expect that this reaction will be useful in organic synthesis.
Typical procedure
The bromohydrin (3.5 mmol) dissolved in benzene (100 ml) or toluene is poured into quartz tubes capped with a septum. p-Toluenesulfonic acid (1.8 mmol) is then added. Argon is bubbled through the solution for 5 minutes. The solution is irradiated using a Rayonnet type system (254 nm) for one to three hours (TLC monitoring). After complete disappearance of the starting material, the solution is filtered to remove p-TsOH. The solvent is distilled and the crude product is purified by flash-chromatography.