Introduction
This indanylamphetamine, (also called IAP, 1-(5-Indanyl)-2-aminopropane or 5-(2-aminopropyl)indane) is an analog of MDA, but with both oxygens in the methylenedioxy bridge replaced by methylene (CH2) units. It has been shown to not be a serotonergic neurotoxin, and is active at 0.2 mg/kg in Nichols' lab rat tests, compared to 0.8 mg/kg for MBDB. However, the lab rat results show that the effects should be somewhere inbetween MDA and MBDB, as it doesn't fully substitute for the purely serotonergic compound MMA (3-Methoxy-4-Methyl- Amphetamine), but does share some of the dopaminergic effects of MDA. Further animal tests showed that IAP does not substitute for amphetamine, so the compound is not a particular stimulant either. IAP is also one of the most active serotonin-releasing agents known so far, together with the 4-iodoamphetamine and 4-methylthioamphetamine (4-MTA). However, 4-iodoamphetamine is a serotonergic neurotoxin, and 4-methylthioamphetamine has also shown signs of toxicity, as it also exhibit MAOI properties. This together with is serotonin-releasing properties, makes it prone to give a serotonin syndrome in people taking it, and several 4-MTA related deaths has been reported. The same cannot be ruled out for IAP, so caution is advised in any human testing of this compound. The animal test results suggest that the active dose is somewhere between 20-40mg. In October 1998, there were news reports that the compound had been seized together with other ecstasy-like compounds in South Australia (possibly Adelaide), confirming that it has been manufactured in clandestine laboratories there.
Don't miss to read this Illustrated Synthesis of IAP By MadMax
Chemistry
Indane is used as a starting material in this synthesis. It can either be bought as is, or made from the ten times cheaper indene by any suitable kind of hydrogenation. The indane is formylated here through Friedel-Crafts alkylation with dichloromethyl methyl ether using Tin(IV)Chloride as the Lewis acid. The intermediate product from the alkylation hydrolyzes to the aldehyde in water. To purify the aldehyde, it is stirred with activated carbon (such as powdered Norite), and then filtered through some silica gel and Celite. The crude aldehyde is pure enough for subsequent reactions described here, but can be purified either by making its bisulfite adduct, washing this, and then decompose it by the addition of concentrated potassium carbonate solution, followed by extraction into dichloromethane and evaporating. It can also be purified by distillation, bp 100-101°C at ~0.2 mmHg. The common Vilsmeier formylation gives a lot of tar when used on indane and gives a mixture of both the 4- and 5-carboxaldehyde. Other formylation strategies for indane has been discussed in Kinetic's Indane Formylation Doc.
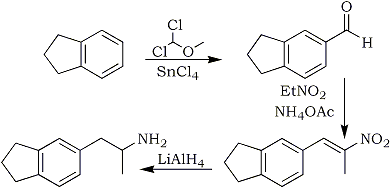
Preparing the nitropropene is pretty straight-forward, just a reflux with ammonium acetate and nitroethane, but the purification is more of a hassle, since it is an oil at normal temperatures, it has to be purified by flash chromatography. The best method seems to be to wash the reaction mixture with water until all the inorganics and nitroethane has been removed, dilute with methylene chloride and dry the solution over MgSO4, evaporate the solvent and running the nitroalkene through a short column packed with silica gel.
The final reduction of the nitropropene and the subsequent isolation of the final indanylamphetamine is pretty straightforward, and the final product can be recrystallized from isopropanol/ether to improve the melting point. The overall yield of IAP from indane is 54% of theory, which is pretty good.
Experimental1
Indane-5-carboxaldehyde
10g (84.6 mmol) of indane was dissolved in 100ml dichloromethane and cooled to 0°C on an ice bath. With vigorous stirring, 15ml (127 mmol) SnCl4 was added all at once via syringe, folllowed by the dropwise addition of 9.72g (7.65ml, 84.6 mmol) dichloromethyl methyl ether over a 10-minute period. After 30 minutes, the ice-bath was removed and the reaction was quenched by the addition of 125ml ice-water. The aqueous layer was discarded, and the organic phase washed with 3x100ml water, 3M HCl (3x50ml) and 2x50ml brine. The organic solution was stirred with activated carbon and filtered through a thin pad of silica gel on Celite. After removal of the solvent, the residue consisted of 12.2g (98%) of a yellow oil pure enough for the next step.
5-indanyl-2-nitropropene
Indane-5-carboxaldehyde (4.0g, 15.9 mmol) was heated at reflux with 30 ml of nitroethane and 1.22g (15.9 mmol) of ammonium acetate for 4h to yield 2.43g (76%) of the nitropropene as a yellow oil which could be crystallized with difficulty from methanol after chromatography (CH2Cl2), mp 33-34°C.
Indanylamphetamine
2.9g (14.3 mmol) of 5-indanyl-2-nitropropene in 100ml dry THF was added slowly to a stirred suspension of 1.5g (35.6 mmol) LAH in 150ml dry THF and the reaction was stirred for 5h at room temperature. Excess LAH was destroyed with the addition of 10ml H2O, the mixture filtered through celite, the filter cake rinsed well with ether and the solvent evaporated on a water bath. The residue was taken up in 100ml ether, extracted with 5x50 ml 3M HCl. The extracts was basified with 25% NaOH and extracted with 3x50ml DCM. The solution was dried over MgSO4, filtered and the solvent evaporated. The residue was taken up in 20 ml ether and gassed with dry HCl gas. Yield after filtering and drying, 2.18g (72%), mp 218-219°C.