In Pihkal1a, Alexander Shulgin mentions that the preparation of 3,4-methylenedioxyphenyl-2-nitropropene can be carried out in cold methanol with aqueous sodium hydroxide as the base1b,1c. In fact, this method is even more reliable, and gives higher yields than the other method advocated by the dear doctor in his book.
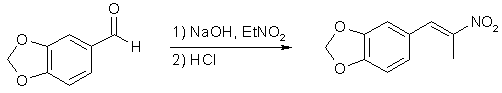
15g of piperonal was dissolved in 40ml of methanol under stirring in a 250ml Erlenmeyer flask. When all of the piperonal had dissolved, 7.1g nitroethane was added to the solution. The flask was put in a ice/salt-bath with magnetic stirring, and when the temperature of the solution had dropped to 0°C, an ice-cold solution of 4g of NaOH in 20ml H2O was added at such a rate that the temperature never rose above 10°C. A white precipitate formed at the bottom of the flask during this addition, which was broken up with a glass rod. The stirring was continued for another hour, while the temperature of the solution was never allowed to rise above 5°C, and at the end of this time, 100 ml of ice-cold H2O was added to the solution, which caused even more precipitation of white solid. The whole slurry was poured into 100 ml of ice-cold 2M HCl solution in a 500ml Erlenmeyer flask, which was gently swirled, and there was a slight bubbling and fizzing, with the color of the solution shifting from white to blue to green to yellow in under a minute. Quite spectacular! When the fizzing had subsided, the solution was once again placed in in an ice-bath with magnetic stirring. When the temperature had dropped to about 5°C, the solution was clear with yellow granules of crude product at the bottom. The granules were filtered with suction, and recrystallized from IPA. After air-drying, the canary-yellow crystals amounted to a yield of 65-70% of theory.
This nitropropene should be used within a week, or stored in the cold, as the color fades to a slight orange over a couple of weeks in room temperature, which is a sure sign of decomposition.
3,4-methylenedioxyphenyl-2-nitropropene2
250 g piperonal is dissolved in 900 ml 95% EtOH and there is added 150 mls nitroethane, 10g methylamine hydrochloride and 8g sodium carbonate. After a brief stirring the mixture is left in a dark place for 2 weeks. It is then poured into 7 L water, the precipitate filtered, washed with water and air-dried. Recrystallization from small qtties of EtOH. Yield 87%.
2,5-Dimethoxynitrostyrene3
A mixture of 2,5-dimethoxybenzaldehyde (1.97 g, 11.8 mmol) and nitromethane (0.72 g, 11.8 mmol) in methanol (200 mL) was stirred at room temperature until the solids dissolved. The solution was cooled to 0°C and a 10.5 M NaOH solution (2 mL) was added dropwise over 20 min. The alkaline solution was added slowly to a 4% HCl solution (200 mL) maintained at 60°C. The pale yellow amorphous solid that formed was filtered and washed with water (200 mL). The crude product was recrystallized from absolute ethanol to give yellow needles (2.11 g, 85%).
Preparation of 10.5 M NaOH:
With cooling, dissolve 10.5g sodium hydroxide in 20ml water, and at RT dilute the solution to exactly 25ml.