Reactive sulfonate esters are especially useful because of their synthetic versatility and ability to initiate carbonium ion reactions. The usual Tipson procedure4 is not suited for the synthesis of reactive sulfonate esters owing to facile alkylation of the solvent, pyridine, by the products5. For the synthesis of propargyl brosylates and tosylates, this side reaction has been suppressed by the use of excess 2,6-lutidine in DCM solution6, and in the case of benzyl tosylates, by reaction of tosyl chloride with the appropriate lithium7 or sodium5 alkoxide. Although very reactive species my be prepared by these procedures, the relatively long reaction times (days) of the former and the strongly basic conditions of the latter seem to limit both procedures to products which are stable to elimination. Another successful procedure for the preparation of reactive tosylates is reaction of the corresponding alkyl iodide with silver tosylate8. Both benzyl and branched chain tosylates may be prepared by this method; however, the stereochemistry of the product is uncertain. Recently Coates and Chen have published a synthesis of reactive tosylates which involves oxidation of the corresponding sulfinates with m-chlorobenzoic acid in DCM solution9. This method appears to have general applicability, although, from the corresponding alcohol, two synthetic steps are required. The method also seems to be restricted to molecules not containing easily oxidized functionalities.
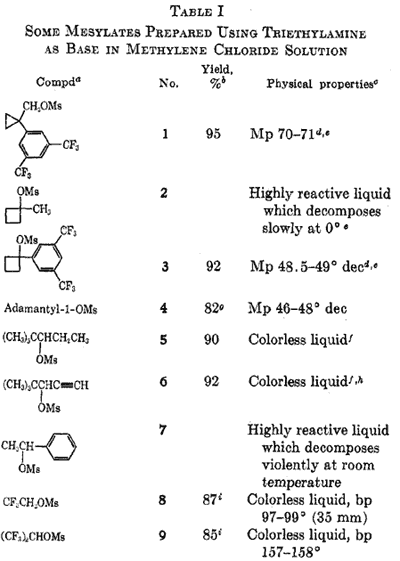
For some time we have synthesized methanesulfonate esters (mesylates) from the corresponding alcohols using a procedure based on the mechanistic studies of Truce10. We wish to report the experimental details of this procedure which is extraordinarily simple and rapid and appears to be of diverse applicability. Table I lists some of the mesylates prepared by this procedure. Repetitive integration of the 60MHz 1H nmr spectra showed that in each case the product was over 95% esterified. No by-products were observed. Our procedure deviates from the usual Tipson procedure4 by the use of triethylamine as base and DCM as solvent. In the light of recent evidence11, it is apparent that the mechanistic course of the reaction has been changed from the usual nucleophilic addition to the alcohol to the sulfonyl group to an addition of the alcohol to the sulfene12 derived from mesyl chloride by the E2 elimination of HCl11. The facile esterification of a number of tertiary and neopentyl systems (Table I) indicates that the reagent has a small steric requirement. The nucleophilicity of the alcohol is unimportant as shown by the ready esterification of 2,2,2-trifluoroethanol and 1,1,1,3,3,3-hexafluoro-2-propanol. Furthermore, conditions are sufficiently mild that even very reactive systems such as 1-methylcyclobutyl13 and alpha-phenyethyl may be esterified. Indeed, in our experience, all alcohols are esterfied by this procedure; the limiting factor seems to be the stability of the product14.
We have found mesylates to be quite useful as synthetic intermediates. They are about three times less reactive towards solvolysis than the corresponding tosylates15. With suitably unhindered systems, mesylates are easily displaced by nucleophiles such as halide or hydride16. In the latter case, where reduction is performed with excess LAH in ether, mesylates are especially useful because the mesylate fragment reduce to methyl mercaptan, which is easily removed.
Experimental
Preparation of Mesylates:
To an approximately 0.2M solution of the alcohol in DCM17 solution containing a 50% molar excess of triethylamine, kept at between 0°C and -10°C, was added a 10% excess of methanesulfonyl chloride (mesyl chloride) over a period of 5-10 minutes18. Stirring for an additional 10-15 minutes completed the reaction. The reaction mixture was transferred to a separatory funnel with the aid of more DCM. The mix was first extracted with ice water, followed by cold 10% HCl acid, sat. sodium bicarbonate, sat. brine. Drying the DCM solution followed by solvent removal gave the product19, which was pure enough for most uses, including solvolysis.