It was demonstrated by the synthesis of pyrocatechol methylene ether that methylenation by means of methylene chloride can be effected more conveniently under normal pressure with the use of a high-boiling solvent1. Benzyl alcohol proved to be the most suitable of the solvents tested for this purpose.
Study of this reaction was continued and yielded some new facts.
Ethylene glycol was chosen as the reaction medium. However, the mere substitution of one solvent for another led to a number of changes in the methylenation method. This was due to differences between the properties of ethylene glycol and benzyl alcohol. Ethylene glycol is not volatile in steam, and at room temperature it is not miscible either with methylene chloride or with pyrocatechol methylene ether. Soda ash is less soluble in benzyl alcohol than in ethylene glycol. The main reaction component, pyrocatechol, is easily soluble in both ethylene glycol and benzyl alcohol.
The first attempt to prepare pyrocatechol methylene ether in ethylene glycol was unsuccessful. The yield of pyrocatechol methylene ether was 10.5%. Probably one of the causes of the unsatisfactory yield was the low temperature (about 100°C) maintained during the reaction. The temperature could not be raised without volatilization of the greater proportion of the methylene chloride which had been put in at the same time before the heating started . The apparatus had to be modified somewhat so that methylene chloride could be fed uniformly into the reaction mixture at 122-124°C. A decrease of the amount of solvent to four times (from ten times) the amount of pyrocatechol was also unfavorable.
The subsequent experiments were conducted in a special apparatus with an attachment (similar to the trap of the Dean and Stark apparatus). With this attachment water could be removed from the reaction zone and methylene chloride vapor could be circulated continuously through the reaction mixture. Potash and soda ash were tested as the alkaline reagents; both gave the same yield of pyrocatechol methylene ether. The yield was the same whether they were added either by portions or in a single operation. Having chosen soda ash, we found that it is more conveniently added before the start of heating.
An attempt was also made to prepare pyrocatechol methylene ether in presence of sodium iodide. However, in this case the maximum yield of pyrocatechol methylene ether was only 45.5% as compared with 38-43% (see table). Since the recovery of sodium iodide is definitely laborious, we consider that it is better to effect methylenation in ethylene glycol without the use of sodium iodide, despite the fact that a lower ether yield is obtained. Ethylene glycol free from moisture is preferable. Generally ethylene glycol is dried over soda ash before use and then distilled in vacuum. Ethylene glycol is easily recovered; the losses in recovery amount to 20-30%. Recovered ethylene glycol is suitable for use in the methylenation reaction, and the yield of pyrocatechol methylene ether obtained with it is 2-3% higher .
Thus, this new version of the "nonautoclave" method of methylenation with methylene chloride differs from the former by giving a higher yield of pyrocatechol methylene ether (38-43% as compared with 32%) and by a simpler procedure for obtaining the methylenation product. Rectification is eliminated, because the pyrocatechol methylene ether distilled with steam contains only small amounts of methylene chloride and water which are removed during the usual vacuum distillation.
The only side product of the reaction we were able to isolate was a very small quantity of a crystalline substance of high melting point. It was isolated only in the course of experiments on a larger scale in a small stainless steel apparatus, during recovery of ethylene glycol. This crystalline substance distilled under vacuum with the last ethylene glycol runnings, so that the distillate became opalescent. Fine crystals were deposited on standing in the receiver containing ethylene glycol. They were filtered off and dried. When dissolved in chloroform they gave a colored solution. The solution was decolorized by means of activated carbon; light shining crystals were deposited on addition of ligroin (bp range 40-60°C). When dried in air, they melted at 260.5°C with decomposition.
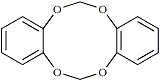
This substance is probably the dimer of pyrocatechol methylene ether, the preparation and properties of which are described in a recent paper2, which also gives its structure:
According to that paper, the dimer of pyrocatechol methylene ether melts at 261.6-262.6°C with decomposition. We were unable to detect any other side products in the preparation of pyrocatechol methylene ether.
In addition to pyrocatechol, we also tested 3-allylpyrocatechol (1,2-dihydroxy-3-allylbenzene) in the methylenation reaction. 3-Allylpyrocatechol is a liquid, thick at room temperature and miscible with ethylene glycol. The reaction conditions were the same as in the preparation of pyrocatechol methylene ether. The yield of the methylenation product, 3-allylpyrocatechol methylene ether (o-safrole) was 51% of the theoretical.
Preparation of o-safrole by methylenation of 1,2-dihydroxy-3-allylbenzene with methylene iodide in presence of potash in acetone solution is described in the literature. The reported yield of o-safrole is 32.4%3.
Experimental
Preparation of Pyrocatechol Methylene Ether
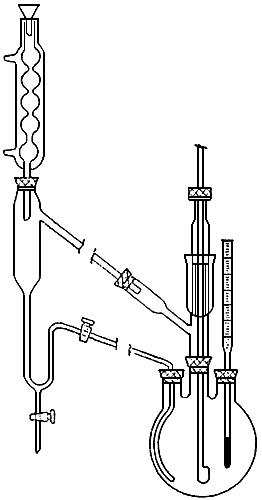
A mixture of 350 g of dry ethylene glycol, 35 g of pyrocatechol, 55 g of dry soda ash, and 18.5 g of methylene chloride was put in a 750-ml round-bottomed flask fitted with a mechanical stirrer in a mercury seal (with a side tube), a thermometer, and a tube (bubbler) reaching to the bottom of the flask. The bubbler tube was connected to the lower tube of the attachment (see figure) which was equipped with a reflux condenser . When the temperature of the reaction mass reached about 80°C methylene chloride began to distill; it condensed in the condenser and collected in the trap. The temperature of the reaction mass gradually rose to 122°C, when the stopcock of the attachment was partially opened to allow methylene chloride to pass into the reaction mixture. Methylene chloride was added at intervals by portions through the condenser into the attachment so that it entered the reaction mass at about 20 g per hour. Water formed in the reaction and a small amount of ethylene glycol distilled together with methylene chloride (two layers are formed in the attachment, with the aqueous layer on top) . The heating time at 122-124°C was from 8 to 12 hours. The amount of methylene chloride added was about 100 g. In the attachment 15 g of aqueous layer containing about 15% of ethylene glycol was collected. When cool, the reaction mass was transferred to a flask for steam distillation. During 25 minutes 22.8 g of reaction product and 680 g of water was distilled. The reaction product was distilled from a Claisen flask with a still head (with six points). First the unchanged methylene chloride (2.8 g) was distilled under normal pressure at 40-50°C, and the residue was then distilled under vacuum. The yield of pyrocatechol methylene ether was 17 g (43.6%); bp 74-75°C at 25 mm, nD20 1.5385.
Preparation of o-Safrole
The apparatus was the same as that used for preparation of pyrocatechol methylene ether. 55 g of soda ash and 18.5 g of methylene chloride was added to a solution of 35 g of 1,2-dihydroxy-3-allylbenzene in 350 g of ethylene glycol. The mixture was heated with stirring. At 122-124°C methylene chloride was fed continuously into the reaction mixture from the attachment through the bubbler tube. Fresh portions of methylene chloride were introduced from time to time into the attachment through the condenser. 80 g of methylene chloride was added during 10 hours of heating. At the end of the reaction 28 g of unchanged methylene chloride and 14 g of aqueous layer was removed from the attachment. When cool, the reaction mass (which was alkaline to phenolphthalein) was put into a flask for steam distillation. During 1 hour 25 g of an oily substance was distilled (the residue from steam distillation was alkaline to phenolphthalein). Vacuum distillation yielded 19.2g (51%) of a colorless substance with bp 108-109.5°C (16 mm) nD20 1.5370. At 749 mm the bp was 223-225°C.
In addition to o-safrole, 1.2 g of a substance with bp 110°C (16 mm), nD20 1.5430 was obtained. This substance was insoluble in 10% caustic soda; its odor resembled that of o-safrole. The higher boiling point and refractive index of this substance may perhaps be due to the presence of a small amount of o-isosafrole which might have been formed from o-safrole by isomerization.
According to literature data3, o-safrole has bp 106-107°C at 16 mm and 226-227°C at 762 mm; nD20 1.5359.
Summary
- Pyrocatechol methylene ether was synthesized by methylenation of pyrocatechol with methylene chloride in presence of soda ash in ethylene glycol without the use of pressure; the yield was 38-43% of the theoretical (calculated on the pyrocatechol taken).
- The same method was used for preparing o-safrole from 1,2-dihydroxy-3-allylbenzene. The yield was 51% of the theoretical, calculated on the 3-allylpyrocatechol (1,2-dihydroxy-3-allylbenzene) taken.