Synthesis of 5-MeO-Tryptamine[ Back to the Chemistry Archive ] Hydrolysis of Melatonin to 5-MeO-tryptamine (Mexamine) [1]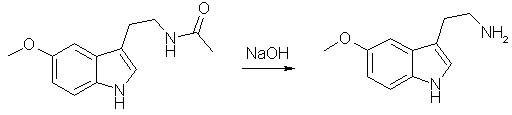
42g melatonin taken up in 300g isobutanol. Add 30g NaOH and 3g sodium dithionite. Reflux 2h @ 105 deg. under N2. Cool mixt. ext with 500g water. Aqueous phase conatins sodium acetate and excess NaOH-seperate. Acidify isobutanol phase to pH 2 with 32% HCl. Concentrate solution to induce crystallization of 36g crude mexamine. Recrystallize from 96% EtOH to obtain 30g white crystalls of mexamine of 98.5% purity. Synthesis of 5-MeO-tryptamine (Mexamine) [2]3-Bromopropylphthalimide Into a 3-neck 1-liter flask, provided with stirrer and cooling medium, there are introduced 101 g (0.5 moles) of 1,3-dibromopropane, 250 ml acetone and 15 g K-phthalimide, by reflux processing the mixture under stirring. At 1 hour time intervals there are added further (15+10+6.3) g K-phthalimide (46.3g corresponding to 0.25 moles), by holding under reflux conditions for a total period of 24 hours. At the end of this period the precipitated KBr is filtered and acetone is evaporated in a rotary evaporating device; the obtained oil is distilled under vacuum (as provided by a water pump) and there are recovered 48.5 g (0.25 moles) of 1,3-dibromopropane, which is distilled at 69-70°C. The residue (dissolved before solidification in the distillation flask) is crystalized twice from ethanol, so as to remove the small amount of formed diphthalimidopropane. There are thus obtained 48.2g (72%) of a white crystaline solid, melting point 72°C. Purity is controlled for TLC on silica gel, using as eluent benzene-acetone (45:5), freshly prepared, Rf of about 0.95 (diphthalimidopropane having a lower Rf). By analogous reactions, in which K-phthalimide is added once, there is obtained a product which contains greater amounts of diphthalimidopropane, thereby it is necessary to purify by distillation (e.g. 150°C./0.25 mm) by using a Vigreux device without cooling, since the distillate tends toward solidification. The yield is substantially equal to the above disclosed yield. Preparing of ethyl-2-acetyl-5-phthalimido-pentanoate In a three-neck flask having a capacity of 500 ml, provided with CaCl2 cooling there are dissolved 4.60 g (0.2 g/A) Na in 100 ml anhydrous ethanol. To the solution, at room temperature, there are added 27.32 (0.21 moles) of acetacetic esther and then, after ten minutes, 40 g of 3-bromopropylphthalimide and, after one hour, further 12.5 g (in total 52.5 g corresponding to 0.196 moles), by holding the reflux processing and continuing for further three hours. At the end of this period, sodium bromide is filtered, the solution is neutralized by 2N HCl and ethanol is evaporated under reduced pressure. The residue is recovered by ether, washed twice with water, dried on anhydrous Na2SO4 and the solvent is evaporated, thereby providing a light yellow oil which is crystalized by dissolving it in a minimum ethanol amount by adding a small amount of ether and upon ageing for a night. There are thus obtained 45 g (yield 72%) of a white crystalline solid, with m.p. 60°C. Upon recrystalisation there is obtained a m.p. of 63°C. Product also crystalizes from benzene-petroleum ether. TLC on silica gel, benzene-acetone (45:5), Rf about 0.70. Purification of 4-anisidine A sample of 4-anisidine, of a very dark colour, is dissolved in an excess of 2N HCl and the solution is repeatedly extracted by chloroform as far as the colour is no longer extracted. The acid solution is boiled by decolorizing charcoal and hot filtered. The strongly cooled filtrate is processed by concentrated NaOH and extracted by chloroform. The chloroform solution is dried on anhydrous Na2SO4 and evaporated under reduced pressure. The residue is crystallized from benzene thereby providing a white lamellae product with a melting point of 57°C. Preparing of 2-carboxyethyl-5-(2-phthalimidoethyl)-5-methoxy-indole 24.64 g (0.2 moles) p-anisidine in 80 ml ethanol, 120 ml water and 80 ml (0.96 moles) 37% HCl are diazotized at 0-5°C. by 14.5 g (0.21 moles) NaNO2 in 40 ml water; at the end the reaction is continued for other 30 minutes at the same temperature. The thus obtained diazonium salt solution is added to a solution (stirred and held at 0°C.) of 63.46 g (0.2 moles) of ethyl-2-acetyl-5-phthalimidopentanoate and of 130.64 g (0.96 moles) of sodium acetate trihydrate in 700 ml ethanol. The reaction is continued for 1 hour (the end pH must be included in the 5-6 range); then the solution is brought to room temperature under stirring for other three hours. At the end of this period, the mixture is diluted with 2 l water and extracted by CH2Cl2 three times; the organic phase, after washing by water and drying on anhydrous Na2SO4, is evaporated, thereby providing 89.2 g of a dark red oil which is dissolved in a minimum amount of ethanol and introduced into a 3-neck 1 liter capacity flask, provided with stirrer, cooler and loading funnel. By stirring and heating there are added in 20 minutes 480 ml of a 10% solution of gaseous HCl in ethanol, by refluxing for 2 hours. At the end of this period, the mixture is cooled down (for a night in a refrigerator or for 3 hours in an ice bath) and filtered by fully washing with methanol, water and methanol again. The dry solid material has a weight of 57.3 g (yield 73%), with a m.p. of 234-237°C. By recrystalisation from glacial acetic acid there are obtained 54.9 g (yield 70%) with m.p. 239-240°C. TLC on silica gel, concentrated benzene-methanol-ammonia (50:10:1), Rf about 0.80. Preparing of 5-methoxytryptamine Into a 3-neck 3 liter flask, provided with stirrer, cooler and loading funnel, there are introduced 58.86 g (0.15 moles) of 2-carboxyethyl-3-2-phthalimidoethyl-5-methoxy-indole and 187.5 ml (15 g; 0.375 moles) of 2N NaOH and the mixture is refluxed at 135°C. for 2.5 hours, thereby providing a complete solution. By holding stirring and temperature, there are added, in 30 minutes, 750 ml of H.sub.2 SO.sub.4 (at 20%) (v/v), by further reflux processing for 4 hours. At the end, the solution is cooled (for a night in a refrigerator or for 3 hours in an ice bath), by removing by filtration the precipitated phtalic acid. The solution is made alkaline by cooling with 30% NaOH and extracted three times with CH2Cl2, the collected extracted materials are washed with water, dried on anhydrous Na2SO4 and evaporated, thereby providing 20.25 g (yield 71%) of crude 5-methoxytryptamine. TLC on silica gel, sat. CHCl3, NH4OH-methanol (50:2), Rf about 0.65. Purifying of 5-methoxytryptamine To purify 5-methoxytryptamine, 19 g (0.1 moles) crude product and 76 ml (58.86-0.36 moles) of hexamethyldisilazane are refluxed for a night in a flask with sodium hydroxide protected cooling. The solution is firstly distilled under normal pressure for recovering excess HMDS (43.6 g; 0.27 moles; m.p. 124-125°C.) and then under a reduced pressure, thereby providing a mixture of biderivative (20.26 g; m.p 135-140°C. at 0.1 Torr) and monoderivative material (5.25 g; m.p. 165°C. at 0.1 Torr). The silyl derivative is hydrolyzed by aqueous methanol, thereby providing 15.36 (0.08 moles) with a yield of 80%. The mixture is crystalized from ethanol, so as to provide a white product having a m.p of 120-121°C. References [1] US Pat 4,772,726
|