Abstract
The combination of zinc chloride and sodium borohydride in dichloromethane is used to effect reductive aminations of formaldehyde with a variety of primary and secondary amines containing potentially acid-sensitive functional groups in good to excellent yields.
Reductive amination1,2 of aldehydes and ketones that allows a direct path from carbonyl compounds to amines is a fundamental reaction in synthetic organic chemistry. Among the hydride reagents, sodium cyanoborohydride3 has been widely employed to effect this transformation in recent years. The other reagents include sodium borohydride in neat liquid carboxylic acid media4 or in aqueous sulphuric acid5, However, the presence of acid-sensitive and easily solvolyzed functionality is limited and the use of highly toxic sodium cyanoborohydride raises the risk of residual cyanide in the product or in the work-up waste system. Development of alternative procedures that would effect the transformation in the presence of a variety of functional groups and obviate the utilization of sodium cyanoborohydride is, therefore, an important objective. From this viewpoint, we have recently reported, the use of zinc chloride and zinc borohydride, a uniquely mild reducing agent , in the reductive alkylation of amines. Based on this concept, itis now reasoned that a more convenient, short and straightforward procedure for reductive amination of aldehydes and ketones can be developed using the combination of zinc chloride and sodium borohydride as a mild, safe and efficient one-pot reagent system. Indeed, this has been the case. Herein, the results for the novel use of this reagent system in the reductive methylation7 of a wide variety of primary and secondary amines containing potentially acid-sensitive functional groups with paraformaldehyde are described (Scheme). This is an interesting development of our previous findings6 which offers further improvement in the conditions for reductive amination reactions. Attempts to effect the transformation with both sodium and zinc borohydrides were unsuccessful in the absence of zinc chloride. Evidently, zinc chloride is functioning as a Lewis acid catalyst as well as an excellent water scavenger to produce intermediate imines which are then reduced by zinc borohydride generated in situ. Zinc chloride has been found to be compatible with a variety of acid-sensitive functional groups including acetals.8
Scheme
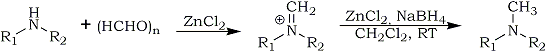
The utility of this method is evaluated by reacting a mixture of paraformaldehyde and various amines containing potentially acid-sensitive or reducible functional groups with zinc chloride and sodium borohydride. The molar ratio of the reactants and the results obtained for a representative group of amines are shown in Table 1. The reducing agent was added only after the mixture of amine, paraformaldehyde and zinc chloride had been stirred for 1 h at room temperature. The reaction mixture was further stirred at room temperature for 8-11 h and then poured into aqueous ammonia. Dichloromethane was the solvent of choice. Drying of the organic extracts and concentration provided N-methylated amines which were purified either by distillation or by flash chromatography. Paraformaldehyde was used as the convenient source of formaldehyde and like other reductive methylation procedures7 the reaction medium was not exposed to protic acids or protic solvents.
Table 1. [Click Link Below to View]
Representative Reductive Methylation of Amines
with ZnCl2 - (HCHO)n - NaBH4 in CH2Cl2 at rt.
The secondary amines afforded the pure N-methylated tertiary amines in excellent yields without any chromatographic separation. Dicyclohexylamine and diisopropylamine which might exhibit steric hindrance to N-substitution were smoothly converted into the corresponding N-methylated tertiary amines in nearly quantitative yields. The reaction conditions were found to be tolerant to a number of groups such as hydroxyl, chloro, carboxylic esters, amide, carbamate and nitro. The primary amines afforded the N,N-dimethylamines in good yields; the crude products were purified either by flash chromatography or by crystallization. The very weak base m-nitroaniline (pka 2.47) was also converted into the corresponding N,N-dimethylated product in good yield. In general, the aromatic primary amines reacted slowly as compared to the aliphatic primary amines. Since the reductive amination of formaldehyde with secondary amines is invariably faster9 than with the primary amines, it has not been possible to N-monomethylate primary amines under these conditions.
The mild aprotic reaction medium that can tolerate a number of acid-sensitive and normally reducible functional groups, easy work-up, the high yields of pure products and the use of cheap, and safe reagents are the significant advantages of the present method over the existing reductive alkylation protocols. This one-pot procedure, thus, represents an efficient, environmentally compatible alternative for the Borch reductive alkylation of amines with highly toxic and expensive sodium cyanoborohydride.
In summary, a mild, safe and efficient alternative to the Borch reductive alkylation of amines is presented that utilizes zinc chloride and sodium borohydride as a one-pot reagent system. This development of our previous findings6 which avoids the preparation of zinc borohydride offers further improvement in the conditions for reductive amination reactions. Work is now underway to extend its application and will be reported in due course.
Experimental
The commercially supplied starting amines were either distilled over KOH or recrystallized from appropriate solvent mixtures prior to use. All reactions were carried out in dichloromethane freshly distilled over calcium hydride. Thin-layer chromatography was done on pre-coated silica gel plates (Aldrich). 1H NMR spectra were measured in CDCl3 solution using TMS as the internal standard. Representative procedures for N-methylation are given below.
Methylation of Dibenzylamine
A mixture of dibenzylamine (0.98 g, 5 mmol), zinc chloride (1.36 g, 10 mmol) and paraformaldehyde (300 mg, 10 mmol) in dichloromethane (25 ml) was stirred for 1h at room temperature under dry atmosphere. Sodium borohydride (0.38g, 10 mmol) was then added to the reaction mixture and sliming was continued for a further period of 8h. The reaction was then quenched with excess ammonium hydroxide (40 mL, 2N) and the whole mixture was stirred for 10 min. The organic layer was separated and the aqueous part was extracted once with dichloromethane (25 mL). The combined dichloromethane extracts were concentrated in vacuo after drying (Na2CO3) to afford a colourless oil which was distilled to give pure N-methyldibenzylamine: yield 0.95g (90%); bp 122°C/0.7mm Hg (lit.7f bp 125°C/0.7mm Hg).
Methylation of Benzylamine
A mixture of benzylamine (0.54g, 5 mmol), zinc chloride (2.7g, 20 mmol) and paraformaldehyde (0.6 g, 20 mmol) in dichloromethane (25 mL) was stirred at room temperature for 1 h at room temperature under dry atmosphere. Sodium borohydride (0.76 g, 20 mmol) was then added and the resulting mixture was stirred for 1h. The reaction mixture was then quenched by addition of aqueous ammonia (40 mL, 2N), stirred for 10 min. and the organic layer was separated. The aqueous part was extracted with dichloromethane (25 mLx1) and the combined organic extracts were concentrated in vacuo after drying over anhydrous Na2CO3. The crude product was purified by flash chromatography over neutral alumina using hexane: diethyl ether (3:1) as the eluent to yield 0.48g (70%) of pure N,N-dimethylbenzylamine as a colourless oil; bp 181-183°C/760mmHg (Lit.7f 185°C/760mmHg).