Abstract
By use of sodium borohydride in N,N-dimethylformamide solution containing a molar excess of pyridine as a borane scavenger, direct conversion of both aliphatic and aromatic acid chlorides to the corresponding aldehydes can be achieved in >70% yield with minimal (5-10%) alcohol formation.
One of the most useful synthetic transformations in organic synthesis is the conversion of an acid chloride to the corresponding aldehyde without overreduction to the alcohol. Until recently, this type of selective reduction was difficult to accomplish and was most frequently effected by catalytic hydrogenation (the Rosenmund reduction1). However, in the past few years, several novel reducing agents have been developed2 to accomplish the desired transformation.
In view of its selectivity as a reducing agent, ease of handling, and low cost, sodium borohydride would seem to be an attractive reagent for effecting the selective reduction of an acid chloride to an aldehyde. However, the latter transformation was consistently reported to occur with overreduction to the alcohol until Johnstone and coworkers3 discovered that the intermediate aldehyde could indeed be isolated in fair to good yield if such reductions are conducted at 0°C in acetonitrile in the presence of certain metal ions complexed with N,N-dimethylformamide (DMF). In the absence of metal salts aldehydes were isolated, albeit in low yield, only if the reduction was effected at -70°C.
Since this metal-assisted borohydride reduction of acyl halides suffers from low to moderate yields (24-58%) obtained with aliphatic compounds and results in a significant amount (always >10%) of alcohol from overreduction, we decided to investigate the reaction further. Our initial studies4 revealed that sodium borohydride in a mixture of DMF-tetrahydrofuran (THF) is capable of converting both aliphatic and aromatic acyl chlorides to the corresponding aldehydes in high yield without the presence of any additional metal salts if the reaction is quenched by use of a mixture of propionic acid - dilute hydrochloric acid - ethyl vinyl ether. Unfortunately, aldehydes could be isolated in good yield using this methodology only if the reaction was conducted at low temperature. For example, treatment5 of o-chlorobenzoyl chloride with sodium borohydride in DMF - THF at -70°C afforded a product mixture containing the corresponding aldehyde and alcohol in a 5:1 ratio. However, a similar experiment6 conducted at 0°C resulted in a substantial amount of overreduction since o-chlorobenzyl alcohol comprised 75% of the product mixture. Somewhat unexpectedly, doubling the reaction time prior to the quench had little effect on the overreduction in the latter reaction an indication that overreduction might be occurring during the quench. Since the procedures used in quenching the reactions at -70°C and 0°C were virtually identical7, this disparity of results offers further evidence8 of the stability of the initial reaction intermediate (1) at -70°C (and lack thereof, with generation of borane, at 0°C).
In addition to the disadvantage of requiring a low reaction temperature, our previous methodology4 presented two other difficulties. The aldehydes in all cases were contaminated with varying amounts of material derived from ethyl vinyl ether; and the unusual quench presented a serious hazard due to the vigorous evolution of hydrogen. This communication reports that such problems can be circumvented if the reduction is effected in the presence of pyridine, which serves as an efficient scavenger of the borane presumably liberated9 if the reaction is conducted at 0°C.
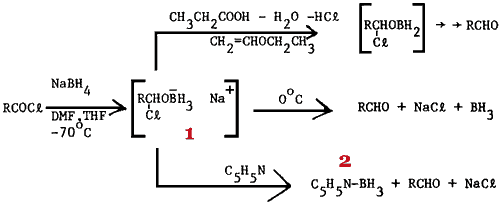
In contrast to the results obtained at 0°C in the absence of pyridine, it was found that treatment of o-chlorobenzoyl chloride with sodium borohydride in DMF-THF containing a molar excess of pyridine using a procedure only slightly different10 from that described below afforded the corresponding aldehyde contaminated with less than 20% of the corresponding alcohol. Accompanying these two products was a substantial amount of pyridine borane (2)11. The apparent stability of the latter compound (2) in the presence of the aldehyde is consistent with the known12 properties of this reducing agent i.e., it reduces aldehydes and ketones only at elevated temperature. Since pyridine borane is virtually insoluble in water and very soluble in ether, a chromatographic separation seemed called for. Unexpectedly, when the aldehyde-pyridine borane mixture was applied neat to a column of Florisil13, rapid elution with 84:16 hexane/ether did succeed in leaving pyridine borane on the column but afforded a 95:5 mixture of alcohol:aldehyde! Equally frustrating were attempts to destroy pyridine borane with strongly acidic quenches (20mL of 2M aqueous hydrochloric acid; 20mL of propionic acid- 9mL of 4M aqueous hydrochloric acid) prior to reaction workup. Such efforts consistently led to a reaction product in which only alcohol could be detected by both NMR and IR analysis! In retrospect, such observations are consistent with the enhanced reducing properties amine borane complexes are known14 to exhibit in the presence of Lewis acids.
Table I
| Starting Acid Chloridea | Yieldb |
Aldehyde:Alcohol Ratioc |
| o-Chlorobenzoyl chloride | 77% | 8:1 |
| Lauroyl chloride | 76% | 12:1 |
| 10-Undecenoyl chloride | 74% | 10:1 |
a) Aldrich Chemical Co., Milwaukee, Wisconsin, U.S.A.
b) This yield is based on the molar amount of NaBH4
utilized for the reaction and refers to distilled product.
c) Determined by NMR (CHO vs. CH2OH signals).
For the aliphatic substrates, product ratios were
determined by GC. Retention times: alcohol>aldehyde.
Eventually a facile method (see the following procedure) was developed to remove pyridine borane in a non-destructive manner from the reaction mixture prior to isolation of the aldehyde. As the results in Table I indicate, this methodology seems to be applicable to both aromatic and aliphatic acid chlorides. A more detailed study of this reduction, including its scope and use of other types of borane scavengers15, is presently being initiated and results will be reported in a future article.
Experimental
Standard Reduction Procedure16
To a solution of 129 mg (3.4 mmoles) of sodium borohydride and 2.0 mL of pyridine in 5.0 mL of DMF and 3.0 mL of anhydrous THF cooled to approximately 0°C (external bath temperature) was added rapidly (<5 seconds) a solution of 4.0 mmoles of an acyl chloride in 2.0 mL of anhydrous THF. This mixture was subsequently stirred at 0°C for 1 minute before 0.50 mL of water was added to the flask to hydrolyze the excess acid chloride. Stirring of this mixture was continued at 0°C for an additional 60 seconds, after which 50 mL of 4:1 (v/v) hexane: solvent ether was quickly introduced into the flask. The globules of pyridine borane which appeared at this point can be separated from the reaction product by rapid filtration of the reaction mixture through a small column of Florisil (25 mL). An additional 25 mL portion of 4:1 (v/v) hexane: solvent ether was used to rinse out the reaction flask and ensure quantitative elution of the desired aldehyde (and any of the corresponding alcohol17) from the Florisil column18. 25 mL of ether was added to the combined filtrate, and this organic phase was washed successively with 15% aqueous NaCl (2x100mL), 1:1 (v/v) 2M aqueous hydrochloric acid:brine (1x100mL), 4:1 (v/v) 1M aqueous NaOH:brine (2x100mL), and saturated brine (100 ml). The organic layer was then dried (MgSO4) and the solvent removed in vacuo.