Table.
Carbonyl Compounds 2 from Nitroalkanes 1
| R1 | R2 | Yielda | Boiling Point |
|
| Found | Ref3 | |||
n-C3H7 |
H |
76% |
72-73°C | 75°C |
n-C4H9 |
H |
81% |
38°C/30mm | 28°C/12mm |
n-C5H11 |
H |
80% |
44°C/9mm | 79°C/86mm |
C6H5 |
H |
78% |
43°C/6mm | 62°C/10mm |
C2H5 |
CH3 |
81% |
76-78°C | 79.6°C |
n-C4H9 |
CH3 |
82% |
37°C/75mm | 127°C |
-(CH2)5- |
88% |
43°C/14mm | 155°C | |
-(CH2)11- |
96% |
mp 58°C | mp 59°C | |
a Yield of isolated product; purity >98%,
determined by 1H-NMR and TLC analysis.
The conversion of primary and secondary nitroalkanes into the corresponding aldehydes and ketones, i.e. the Nef reaction, is a transformation of substantial synthetic utility. The original reaction involves solvolysis of alkali nitronates (i.e. aci-nitroalkanes) with aqueous or alcoholic acid solution.
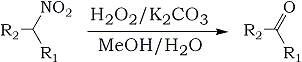
Subsequent variations include reductive [Ti(III), V(II)] and oxidative [O3, O2, RONO, KMnO4 - MgSO4, NaMnO4 - borate buffer, (NH4)2S2O8, t-BuOOH - VO(acac)2] conditions1,2 as well as solid state reactions (sodium methoxide-impregnated silica gel). We have now found a improved method for oxidative Nef reactions. Primary and secondary nitroalkanes (cycloalkanes) 1 on treatment with 30% hydrogen peroxide and potassium carbonate in methyl alcohol solution are smoothly converted into the corresponding carbonyl compounds 2 in good to excellent yields. Results are summarized in the Table. The reaction occurs at room temperature, thus minimizing oxidative side reactions.
Experimental
Carbonyl Compounds 2 from Nitroalkanes 1
General Procedure:
To a stirred solution of the corresponding nitro compound 1 (10 mmol) in methanol (50 ml), cooled to 0°C, is added 30% hydrogen peroxide (20 ml), followed by a solution of potassium carbonate (8 g) in water (25 mL). The stirring is continued for 8 h at room temperature. The solution is then acidified with dilute hydrochloric acid (50 ml) and extracted with dichloromethane (3x20 ml). The combined organic layers are dried with anhydrous sodium sulfate and the solvent is removed under reduced pressure to give almost pure carbonyl compound 2, further purified by distillation or recrystallization, and characterized by 1H-NMR, IR, and TLC analysis (1:1 hexane/benzene).
We consider that the present method is a useful addition to the existing ones due to its simplicity and convenience of high yields under mild conditions.