Abstract
α,β-Unsaturated nitroalkenes are rapidly reduced by chromium(II) chloride to oximes.
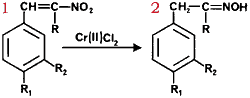
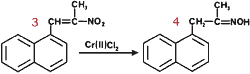
We have been investigating the utility of readily accessible α,β-unsaturated nitroalkenes as precursors to a variety of useful synthetic intermediates such as nitroalkanes1, N-substituted hydroxylamines2, amines3, ketones4 and chromenes5. Recently, we reported that α-alkoxy and α-alkylthio oximes6 and their corresponding ketones7 can be synthesized from conjugated nitroalkenes via a stannous chloride reduction in non-aqueous media. In connection with these studies, we became interested in synthesizing α-hydroxy oximes.
A recent report8 described an unusual reduction of 3-nitroflavenes by chromium(II) chloride to flavonols which presumably occurred via an α-hydroxy oxime intermediate.
Table I
Reduction of Nitroalkenes to Oximes
with Chromium(II) Chloride
Producta |
Yieldb |
Substituents |
Ref |
2a |
15% | R = R1 = R2 = H | |
2b |
70% | R = CH3 ; R1 = R2 = H | |
2c |
68% | R = CH3 ; R1 = Br; R2 = H | - |
2d |
62% | R = CH3; R1 = R2 = OC2H5 | - |
4 |
71% |
- Product exhibited physical and spectral
properties in accord with assigned structures. - Isolated and unoptimized yields;
products are thick oils except 2c, mp 90-91°C.
In addition, it has been reported that a few steroidal nitroalkenes9 lead to the formation of α-hydroxy oximes upon reduction with chromium(II) chloride10-13. We decided to explore the use of chromium (II) chloride in the reduction of β-aryl, α,β-unsaturated nitroalkenes. However, when the reductions were carried out as described in the literature9-10, the main reaction products were the corresponding saturated ketones with trace amounts of α-hydroxyketones; no oxime derivatives were detected. When the reactions were run under milder conditions (room temperature, 10 min) the major products obtained were oximes contaminated with trace amounts of ketones and α-hydroxyketones. None of the expected α-hydroxy oximes were formed!
We thus conclude that chromium(II) chloride rapidly reduces β-aryl, α,β-unsaturated nitroalkenes to oximes rather than α-hydroxy oximes. The reactions can be carried out at room temperature and thus provide a rapid new route to a variety of oximes. The yields are independent of the source of chromium(II) chloride; we find that the use of commercially available material is most convenient. The reactions appear to be most useful for the syntheses of ketoximes. The reduction of β-nitrostyrene to the corresponding aldoxime is accompanied by significant polymerization of the starting material. Our results are summarized in Table I.
Experimental
Melting points are uncorrected. Commercially available samples of 1-nitro-1-cyclohexene, β-nitrostyrene (Aldrich) and anhydrous chromium(II) chloride (Alfa) were used as received. Other nitroalkenes were prepared via published procedures2,14.
Synthesis of Oximes. General Procedure.
The synthesis of phenylacetone oxime is representative of the procedure employed.
Chromium(II) chloride (32 mmol, 3.93 g) was placed in a nitrogen-flushed, 100 mL flask, equipped with a septum inlet and magnetic stirring bar. Aqueous hydrochloric acid solution (3%, 40 mL) was injected into the reaction flask via a syringe; this was followed by the slow addition of a solution of β-methyl-β-nitrostyrene (2 mmol, 0.326 g in 5 mL THF) at room temperature. A moderately exothermic reaction ensued. The mixture was stirred for 10 min, saturated with sodium chloride and the product extracted with ether (3x35 mL). The combined ethereal extracts were washed successively with 5% aqueous sodium bicarbonate and water, dried over anhydrous MgSO4, and the solvent removed under reduced pressure. The crude product (0.27 g) was chromatographed over silica gel column; elution with ether/petroleum ether (1:10) afforded 0.21 g (70%) of phenylacetone oxime15 as an oil17.
(E)-1-(4-Bromophenyl)-2-propanone Oxime (2c)
The reaction was carried out as described in the general procedure to yield 0.31 g (68%) of the product as colorless needles (ether-petroleum ether), mp 90-91°C.
(E)-1-(3,4-Diethoxyphenyl)-2-propanone oxime (2d)
The reaction was performed as described in the general procedure to yield 0.296 g (62%) of the product as a pale yellow oil.