Abstract
α,β-Unsaturated nitroalkenes are readily reduced to the corresponding oximes in good yields using ammonium formate in the presence of palladium. The reactions occur rapidly at room temperature in a solvent system of methanol and tetrahydrofuran.
Catalytic transfer hydrogenation has found widespread use in the reduction of a variety of functionalities.1-3 The ammonium formate/palladium-on-carbon system is reported4 as being a very versatile, selective and rapid method for catalytic hydrogenolysis and has previously been used for the reduction of hydroxyflavones5, isoflavones6, reductive cyclization of 2-nitro-β-nitrostyrenes to indoles7, deoxygenation of heteroaromatic N-oxides8, dechlorination of mono- and polychlorinated aryl compounds9, reduction of azides10 and nitro compounds11,12 to the corresponding amines, reduction aralkyl ketones13, conversion of arylcyano to arylmethyl groups14 and debenzylation15,16 of O- and N-benzyl derivatives.
In a review on catalytic transfer hydrogenation using ammonium formate4, brief mention was made of the unsuccessful reduction of β-nitrostyrene. The authors, however, did not study the reduction of other α,β-unsaturated nitroalkenes. Based on our previous experience with nitroalkenes,17-20 we initiated a study of the hydrogen transfer reduction of α,β-unsaturated nitroalkenes using ammonium formate and palladium-on-carbon.
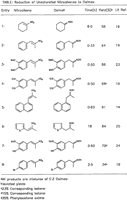
Table 1. [Enlarge]

We wish to report that 1-nitro-1-cyclohexene and a variety of 1-aryl-2-nitro-2-propenes were readily reduced to the corresponding ketoximes in good yields at room temperature in a solvent mixture of methanol and tetrahydrofuran using ammonium formate in the presence of 5% palladium-on-carbon. Our results are summarized in the Table. The expected dehalogenation9 occurred in the reduction of halogen-substituted nitroalkene (entry 8) and the product obtained was a mixture of p-bromophenylacetone oxime and phenylacetone oxime. Ketone formation was observed as a side reaction presumably involving hydrogenolysis of the ketoxime to an imine followed by hydrolysis to the corresponding ketone.21 As anticipated, the reduction of β-nitrostyrene resulted in a complex mixture of products.4
Experimental
Commercially available (Aldrich) samples of 1-nitro-1-cyclohexene, β-nitrostyrene and 5% Pd/C were used as received. Other nitroalkenes were prepared via published procedures.22 Anhydrous methanol and tetrahydrofuran was used and ammonium formate (Aldrich) was dried prior to use. All glassware was oven-dried. All products were characterized by their spectral characteristics (1H-NMR, 13C-NMR and IR)23,24,25
Reduction of α,β-Unsaturated Nitroalkenes. General Procedure:
Dry ammonium formate (315 mg, 5.00 mmol) was added to a solution of nitroalkene (1.00 mmol) in a mixture of tetrahydrofuran and methanol (8 mL, 1:1, v/v). 5% Palladium-on-carbon (236 mg, 10 mol% Pd) was added to the solution, and the resulting mixture stirred at room temperature until all the starting material had been consumed (TLC). The catalyst was removed by filtration and washed with methylene chloride (10 mL) followed by absolute ethanol (10 mL). The solvent was removed under reduced pressure and the residue was washed with water (10 mL) and the product was extracted into methylene chloride (2x20 mL). The crude product was purified by chromatography on a silica gel column using methylene chloride to elute the ketone and absolute ethanol to elute the oxime.