Numerous methods have been employed to prepare nitrostyrenes from the corresponding benzaldehydes. Yields range from low to excellent and sometimes the reaction seems to be capricious and give varying results both as to yield and quality of the product.
Shulgin (PiHKAL) used the ammonium acetate method and reports good results for 2,5-dimethoxy-4-ethylthio and 4-(n)propylthio-β-nitrostyrenes when other researchers (unpublished data) got impure products with this procedure.
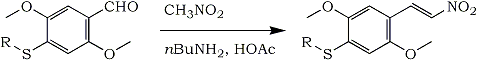
I´d like to publish a modification of the original method, which has proven to give consistently excellent results with these two nitrostyrenes and which should be of general interest for anyone working in this field:
Experimental
Place the starting materials in the following order in a 250 ml round-bottomed flask equipped with a stirring bar and a reflux condenser:
- 0.10 Mole Aldehyde
- 21 mL (0.392 mole) Nitromethane
- 2.0 g (0.033 mole) Glacial Acetic Acid
- 0.59g (0.008 mole) n-Butylamine
Immerse the flask up to three quarters in an oil bath preheated to 90°C and start stirring as the starting materials form a solution.
Raise the temperature up to 95 to 100°C within 10 minutes then stir for a maximum of another 10 minutes at this temperature. Can check for disappearance of starting aldehyde with tlc but be fast! Do not heat until last traces of aldehyde have disappeared as by this time too many side-products will have formed.
Workup by any of the following two methods:
Pour on ice (250g), extract with DCM or CHCl3, wash organic phase with water, evaporate solvent on a water bath, can wash residue with MeOH as this will remove a lot of the impurities without affecting the yield much. Then recrystallize.
Or:
Cool reaction mixture below 60°C, add 150 ml MeOH, stir cold, filter precipitated product with suction, wash with MeOH, recrystallize.
This method takes advantage of the seemingly superior catalytic properties of alkylamine acetate over ammonium acetate.
Alkylamines other than n-butylamine work probably as well (not checked).
It requires less nitromethane than the reported procedure in the literature and gives a very pure product.
The amount of nitromethane could probably reduced even further as long as there still will be a homogenous reaction mixture at reflux temperature.
The amount of amine in the catalyst should remain in the range of 0.05 to 0.08 equivalents in relation to the aldehyde. More amine tends to give more byproducts.
It has specifically been checked for 2,5-dimethoxy-4-ethylthio and 4-(n)-propylthio-β-nitrostyrene but should be applicable also for other substituted benzaldehydes as well as for the higher homologues (nitropropenes and nitrobutenes).