Abstract
Treatment of alkenes (1a-5a) with sodium nitrite and iodine in ethyl acetate (or ether) and water in the presence of ethylene glycol (or propylene glycol) provides conjugated nitroalkenes (1b-5b) in 49-82% yields.
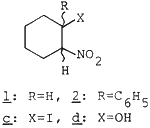
Conjugated nitroalkenes are very useful synthetic intermediates1 and the preparation of the nitroalkenes involves condensation of aldehydes (or ketones) with nitroalkanes or reaction of alkenes with nitryl halides or dinitrogen tetraoxides.2-3 Recently various new approaches for preparation of the nitroalkenes have been reported.1,4-6
In this letter, we wish to report a new and practical method for the preparation of the nitroalkenes from alkenes using sodium nitrite and iodine in ethyl acetate (or ether) and water in the presence of ethylene glycol (or propylene glycol).
Experimental
A typical procedure is as follows:
To a solution of sodium nitrite (1324 mg, 19.2 mmol) and ethylene glycol (893 mg, 14.4 mmol) in 2.0 ml of water, a solution of styrene, 3a (500 mg, 4.8 mmol) in 15 ml of ethyl acetate was added followed by addition of iodine (1828 mg, 7.2 mmol) at 0°C. The reaction mixture was stirred at room temperature for 48 h under nitrogen and then was poured into a separatory funnel together with ethyl acetate and partitioned. The ethyl acetate solution was successively washed with water, aqueous 10% thiosulfate and aqueous sat. NaCl. After drying over anhydrous magnesium sulfate, the ethyl acetate solution was evaporated to obtain crude products. These were purified by silica gel column chromatography eluting with a hexane-ethyl acetate mixture (3:1) to afford pure β-nitrostyrene, 3b in 81% yield. The reactions using other alkenes were performed in the analogous manner and the results were summarized in the Table 1.
Table 1
Preparation of Conjugated Nitroalkenes
| Alkene | Solv.a |
Rxn Cond. |
Productb | Yield |
| Cyclohexene (1a) | A | rt, 96 h | 1-Nitrocyclohexene (1b) | 72% |
| 1-Ph-cyclohexene (2a) | A | rt, 72 h |
1-Ph-2-nitrocyclohexene (2b) | 49%c |
| C6H5CH=CH2 (3a) | A | rt, 48 h | C6H5CH=CHNO2 (3b) | 81% |
B | rt, 48 h |
79% | ||
| C6H5CH2OCOCH=CH2 (4a) | B | rt, 72 h | C6H5CHOCOCH=CHNO2 (4b) | 92% |
1,2-Dihydronaphtalene (5a) |
A | ref, 48 h |
3-Nitro- 1,2-dihydronaphtalene (5b) |
78% |
- 1,2-Diol-Solvent: HO(CH2)2OH-EtOAc (A). HOCH(CH3)CH2OH-Et2O (B).
- All products gave satisfactory spectral data.
- In the case of 2a. 2b and 3d were formed in 49 and 38% yields, resp.
Treatment of 2d with SOCl2-pyridine-Et2O (0°C, 2h) gave 2b in 87% yield.
In the case of 1a and 2a, an iodonitro compound 1c7 (5%) and hydroxy nitro compounds 1d (14%) and 2d (38%) were detected. In the formation of 1c, it is suggested that the nitroalkenes would be formed via the iodo nitroalkanes. Although the exact formation of 1d and 2d is not clear, it is considered that they might originate from 1a, 2a; 1b, 2b and/or 1c, 2c. In the absence of the 1,2-diol, as may be expected from Cornforth's report,8 iodohydrins were obtained as major products. Therefore, the high yield of the nitroalkenes seems to be attributed to the 1,2-diol. The diol strongly interacts with water which is nearly saturated with sodium nitrite. Therefore, the diol might inhibit generation of hypoiodous acid but accelerate that of nitryl iodide. This method is an efficient one-pot procedure, not involving treatment with base. Furthermore, sodium nitrite, cheaper than silver nitrite, could be used for the generation of nitryl iodide. Corey's nitro-mercuration procedure was not appropriate to the nitration of styrene 3a because of ring nitration.9 Recently, Sy has reported for the preparation of 3b with AgNO2-I2-pyridine-Et2O with 45% yield,9 but 3b was obtained in 81% yield in our experiment. Thus, this method is very practical as a synthetic method for the conjugated nitroalkenes from alkenes. Studies on the detailed mechanisms and the scope and limitations of this method are in progress.