Catalytic hydrogenation of β-nitrostyrenes over palladium on charcoal in EtOH-HCl at low temperature gave the corresponding phenethylamines in high yield. Generally, phenethylamines have been prepared by lithium aluminium hydride1 and borane2 reductions of β-nitrostyrenes, which are readily prepared by condensation of the corresponding benzaldehydes with nitromethane. However, such methods are not applicable for large scale preparation. The preparation of phenethylamines by catalytic hydrogenation of β-nitrostyrenes3 reported so far is not convenient, because the reactions have been carried out in acid solutions such as acetic acid with concd sulfuric acid3a or concd hydrochloric acid3b. Tedious treatments and severe reaction conditions such as 50-80°C under 30-100 atm hydrogen3c are also required. We wish to report a convenient catalytic hydrogenation of β-nitrostyrenes under mild conditions.
Typically, a mixture of β-nitrostyrene, 5% palladium on charcoal [K-type (see Experimental), 0.1 equiv] and 12 M hydrochloric acid (2.5 equiv, 1M) in ethanol was stirred under hydrogen (1 atm) at 0°C for 3 h. The representative results are shown in Table 1. The hydrogenations of 3,4-methylenedioxy-β-nitrostyrene (1), 4-hydroxy-3-methoxy-β-nitrostyrene (5), and 3-hydroxy-4-methoxy-β-nitrostyrene (7) at 0°C gave higher yields of the corresponding phenethylamines in comparison with those at room temperature. The low yields of phenethylamines at higher temperature might be due to the palladium induced formation of imine metal hydride intermediates4.
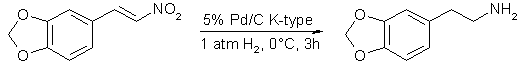
The hydrogenation of 3,4-bis-(benzyloxy)- β-nitrostyrene (9) gave the corresponding debenzylated dopamine hydrochloride (10) quantitatively. It is noteworthy that 3,4-dihydroxy-β-nitrostyrene, which is the precursor of 10, is hardly accessible by the conventional methods. Similarly, hydrogenation of 4-benzyloxy-β-nitrostyrene (11) gave tyramine hydrochloride (12) in 94% yield. Pure alkoxy-substituted phenethylamines can be obtained by simple treatment of the reaction products with hydrochloric acid and subsequently with alkali. The amine hydrochlorides, which are soluble in methanol, such as 10, can be purified simply by single recrystallization.
Table 1
Hydrogenation of β-nitrostyrenesa
| Substrate | Productb | Yieldc |
| 3,4-Methylenedioxy-NS | 3,4-Methylenedioxy-PEA | 71% |
| 3,4-Dimethoxy-NS | 3,4-Dimethoxy-NS | 73% |
| 3-Methoxy-4-Hydroxy-NS | 3-Methoxy-4-Hydroxy-PEA | 81% |
| 4-Methoxy-3-Hydroxy-NS | 4-Methoxy-3-Hydroxy-PEA | 91% |
| 3,4-Dibenzyloxy-NSd | 3,4-Dihydroxy-PEA | 99% |
| 4-Benzyloxy-NS | 4-Hydroxy-NS | 94% |
| 3,4,5-Trimethoxy-NS | 3,4,5-Trimethoxy-PEA | 65% |
- The procedure was described in the text.
- Satisfactory data of IR and NMR were obtained.
- Isolated yields of pure amines or amine HCl.
- Room temperature, 24 h.
The phenethylamines thus obtained can be readily converted into the corresponding 1,2,3,4-tetra- hydroisoquinolines by Pictet-Spengler reaction5. Typically, amine 4 can be readily converted into 6,7-dimethoxy-1,2,3,4- tetrahydroisoquinoline (15) in 80% yield. Furthermore, the secondary amines thus obtained can be readily converted into either nitrones6 or imines7 upon treatment with hydrogen peroxide in the presence of Na2WO4 catalyst or treatment with tert-butyl hydroperoxide in the presence of RuCl2(PPh3)3 catalyst. Typically, the amine 15 can be converted into either 16 or 17 by changing the catalytic system utilized.
Experimental
Materials
5% palladium on charcoal (K-type) was purchased from Nippon Engelhard, Ltd. 3,4-Methylenedioxy-β-nitrostyrene, 3,4-dimethoxy-β-nitrostyrene (3)1b, 4-hydroxy-3-methoxy- β-nitrostyrene (5)1a, 3-hydroxy-4-methoxy-β-nitrostyrene (7)1a, 3,4-bis(benzyloxy)-β-nitro-styrene (9)1b, 4-benzyloxy-β-nitrostyrene (11)1b, and 3,4,5-trimethoxy-β-nitrostyrene (13)1b were prepared by the methods described in the literature.
General Procedure for the Catalytic Hydrogenation.
As a typical example, the preparation of 3,4-methylenedioxy-β-phenethylamine (2) is described.
In a 30 mL side-arm flask equipped with a magnetic stirring bar were placed 3,4-methylenedioxy-β-nitrostyrene (1) (0.500 g, 2.59 mmol), 5% palladium on charcoal (K-type) (0.553 g, Pd 0.26 mmol), 12 M hydrochloric acid (0.5 mL), and ethanol (10 mL). The reaction mixture was stirred at 0°C for 3 h under a hydrogen atmosphere (1 atm). The catalyst was removed by filtration through Celite and washed with ethanol (40 mL). Evaporation of the filtrate gave a yellow oil which was dissolved in water (40 mL), and the solution was washed with CH2Cl2 (20mL x 3). The aqueous layer was neutralized with aqueous ammonia solution (28%, 5 ml.), and extracted with CH2Cl2 (20 mL x 4). The combined organic layer was dried over Na2SO4. Evaporation of the solvent gave 2 (0.303 g, 71%): mp 214.5-216°C (HCl salt) (lit8 mp 209°C).
For the preparation of the phenethylamines bearing a hydroxyl group, the purification procedure was modified slightly. Typically, 4-hydroxy-3-methoxy-β-phenethylamine hydrochloride (6) was isolated without the neutralization with aqueous ammonia (0.422 g, 81%): mp 213.5-215°C (lit.1a mp 213-214°C);
3,4-Dimethoxy-β-phenethylamine (4)
The hydrogenation of 3,4-dimethoxy-β-nitrostyrene (3) (0.500 g, 2.39 mmol) at room temperature for 24h gave 4 (0.316 g) in 73% yield: mp 154-155°C (HCl salt) (lit.9 mp 154-155°C).
3-Hydroxy-4-methoxy-β-phenethylamine Hydrochloride (8)
The hydrogenation of 3-hydroxy-4-methoxy-β-nitro-styrene (7) (0..500 g, 2.56 mmol) gave 8 (0.473 g) in 91% yield: mp 206.5-207 °C (lit1a mp 206-207°C).
3,4-Dihydroxy-β-phenethylamine Hydrochloride (10)
The hydrogenation of 3,4-bis-(benzyloxy)-β-nitrostyrene (9) (0.500 g, 1.38 mmol) at room temperature for 24 h gave 10 (0.260g) in 99% yield: mp 248-250 °C (lit10 mp 240-241°C (dec.)).
4-Hydroxy-p-phenethylamine Hydrochloride (12)
The hydrogenation of 4-benzyloxy-β-nitrostyrene (11) (0.500 g, 1.96 mmol) gave 12 (0.319 g) in 94 % yield: mp 270-272°C (lit11 mp 263-265 °C).
3,4,5-Trimethoxy-β-phenethylamine (14)
The hydrogenation of 3,4,5-trimethoxy-β-nitrostyrene (13) (0.400 g, 1.67 mmol) gave 14 (0.228 g) in 65% yield: mp 184-184.5°C (HCl salt) (lit.12 mp 184-185.5°C);