Regioselective Bromination of Aromatics Using KBr/OxoneSynth. Comm. 32(15), 2313-18 (2002)[ Back to the Chemistry Archive ]Halogenated aromatic compounds are a useful class of intermediates as they are precursors to a number of organometallic species useful in the synthesis of natural products and pharmaceutically important compounds. The manufacture of a range of bulk and fine chemicals, including flame retardants, disinfectants and antibacterial and antiviral drugs, involves bromination. (1) Bromo aromatics are widely used as intermediates in the manufacture of pharmaceuticals, agrochemicals and other specialty chemical products. Consequently, a variety of methods for the bromination of aromatics have been reported in the literature. (2-13) Traditional methods of aromatic bromination involve the use of non-selective hazardous acidic reagents such as mineral acids and metal halides, which can lead to separation difficulties and unacceptable levels of toxic and corrosive waste. Conventional bromination methods typically use elemental bromine, a pollutant and a safety and health hazard. To overcome these difficulties some researchers have utilized a combination of hydrobromic acid and suitable oxidant such as tert-butylhydroperoxide or hydrogen peroxide. (13-15) The replacement of such reagents by non-toxic and more selective reagents is very desirable and represents an important goal in the context of clean synthesis. We report a highly para selective method for bromination of aromatic compounds based on the use of KBr as a bromine source and Oxone® as an oxidant (Scheme 1). 
Potassium peroxymonosulfate is an inexpensive and readily accessible oxidizing agent. It is commonly used as Oxone® (2 KHSO5·KHSO4·K2SO4) and is a versatile oxidant for the transformation of a wide range of functional groups. (16) A number of different aromatic substrates were subjected to the bromination reaction to test the generality of this method and the results are summarized in Table 1 . Efficient bromination of aromatic substrates with good yields and regioselectivity observed with acetonitrile, methanol as solvents (Table 1). As Table 1 shows that the reaction gives high yields and para-selectivity for a range of substituted benzenes of high activity. The results in Table 1 indicate that activated aromatic compounds are more selective for nuclear bromination. Introduction of an electron- withdrawing group on the aromatic ring substantially decreases the rate of ring bromination (Table 1 , Entry 8) while on electron donating group increases it. These system yield selectively para brominated aromatics unless the para-position (Table 1 , Entry 7) is substituted. The para substituted aromatics were brominated in the ortho-position. 2-Methoxy-naphthalene gives 1-bromo-2-methoxy naphthalene. When the less reactive aromatics such as bromobenzene, nitrobenzene, benzoic acid failed to undergo bromination under the same reaction conditions. A wide range of solvents have been employed in these reactions including, carbon tetrachloride, hexane, dichloromethane, methanol and acetonitrile. The best results were obtained when acetonitrile, methanol were used as solvents compared to others (Table 2). The reaction is very fast in methanol compared to acetonitrile. 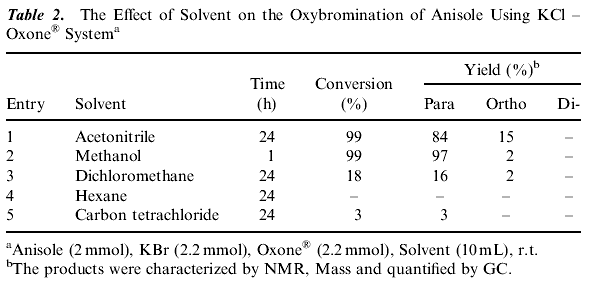
We surveyed the oxybromination with various oxidants such as Oxone®, tert-butylhydroperoxide, hydrogen peroxide and molecular O2. Reactions were conducted with anisole as a probe-substrate at room temperature in acetonitrile. However, Oxone® is far superior to the other reagents. The role of Oxone® was confirmed by conducting a blank experiment where the formation of bromo compound was not observed. Oxybromination of an aromatic compound in the presence of Oxone® proceeds according to the stoichiometry of Eq.1. It is believed that the bromination proceeds via the formation of hypobromous acid. The hypobromous acid has higher instability due to pronounced ionic nature and thus more reactivity towards the aromatic nucleus. (1) ArH + KBr + 2 KHSO5·KHSO4·K2SO4 (2) 2 KHSO5·KHSO4·K2SO4 + KBr (3) 2 HOOSO3K (4) KBr + 2 ·OH + ·OSO3K (5) ArH + HOBr Oxone®, in direct comparison, has a higher onset of decomposition than hydrogen peroxide and liberates less energy. This reaction is performed at lower temperature, which provides a larger margin of safety. Additionally Oxone® is a solid, allowing for the addition of precisely weighed amounts of reagent to be used in the reaction. In conclusion, we have developed a practical method using Oxone® as an interesting alternative to hydrogen peroxide in the oxidative bromination of aromatic compounds. The commercial availability of the reagent, simple reaction conditions, no evaluation of hydrogen bromide and excellent yields of monobrominated products make our method valuable from a preparative point of view. A wide range of solvents has been used in these reactions including carbon tetrachloride, hexane, dichloromethane, methanol and acetonitrile. The best results were obtained by using methanol as a solvent compared to others. We surveyed the iodination with various oxidants such as Oxone®, tert-butylhydroperoxide, hydrogen peroxide and molecular O2. However, Oxone® is far superior to the other oxidants. The role of Oxone® was confirmed by conducting a blank experiment where the formation of iodo compounds was not observed. A typical oxyiodination of an aromatic compounds in the presence of Oxone® proceeds according to the stoichiometry of Eq. (1). It is believed that the iodination proceeds via the formation of hypoiodous acid. The hypoiodous acid has higher instability due to pronounced ionic nature and thus more reactivity towards the aromatic nucleus. The absence of iodination of the ring methyl group (Table 1, Entries 11, 12 and 13) is indicative of the electrophilic mechanism of the reaction rather than a radical pathway. Oxone®, in direct comparison, has a higher onset of decomposition than hydrogen peroxide and liberates less energy. This reaction is performed at lower temperature, which provides a larger margin of safety. Additionally Oxone® is a solid, allowing for the addition of precisely weighed amounts of reagent to be used in the reaction. In conclusion, we have developed a novel system using Oxone® as an interesting alternative to hydrogen peroxide in the oxidative iodination of aromatic substrates. The commercial availability of the reagents, simple reaction conditions, no evolution of hydrogen iodide, high yields of monoiodinated products and work-up is simple. The absence of side chain iodination products in reaction conducted in methanol suggests a substantial increase in the rate of the ionic process. General Procedure for the Bromination of Aromatic CompoundsOxone® (2.2 mmol) was added to a well stirred solution of KBr (2.2 mmol) and substrate (2.0 mmol) in methanol (10 mL) and the reaction mixture was allowed to stir at room temperature. The reaction was monitored by thin layer chromatography (TLC). After the completion of the reaction. The mixture was filtered and solvent evaporated under reduced pressure. The products were purified by column chromatography over silica gel and confirmed by 1H NMR and Mass spectra. 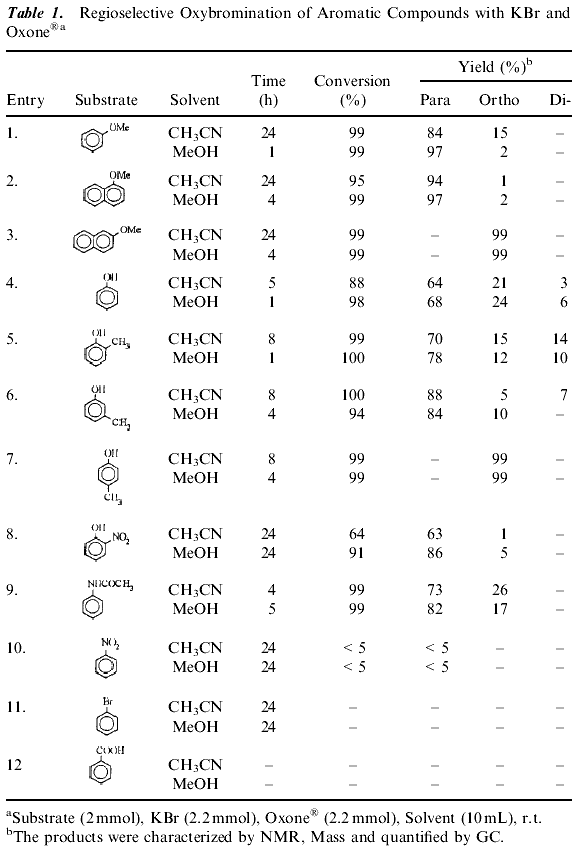
References [1] Ullmann's Encyclopedia of Industrial Chemistry, 6th Ed., Wiley-VCH, Weinheim, 1998. Electronic Release.
|
 ArBr + KOH + K2S2O8·KHSO4·K2SO4 + H2O
ArBr + KOH + K2S2O8·KHSO4·K2SO4 + H2O