Phenyl-2-Propanone from Ephedrine Derivatives[ Back to the Chemistry Archive ] Phenyl-2-Propanone from ephedrines via acid hydrolysis [1]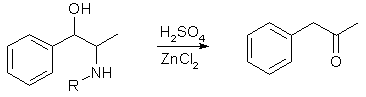
Patent Claims
Ephedrine derivatives that can be used in this procedure include Ephedrine, Pseudoephedrine, Norephedrine and Norpseudoephedrine (Phenylpropanolamine). Example 1 1025g 75% sulfuric acid was mized with 1g ZnCl2, and 192g (1.16 mol) Ephedrine or Pseudoephedrine freebase was dissolved at a temperature of 50-100°C, and the reaction mixture was heated further to 145-150°C. At 125°C steam is passed through the solution to facilitate mixing of the contents. At 145°C the stream of steam is increased, and during 2.5-3 hours the phenylacetone is steam distilled from the reaction mixture. The the crude phenylacetone, which is free from propiophenone is isolated by toluene extraction of the distillate. After distillation through a short vigreaux column, 130g (82%) of phenylacetone is isolated in a purity of 99.8%. Example 2 100g (-)-Pseudoephedrine freebase was dissolved in 1000g 79% sulfuric acid, whereby an acid concentration of 65-66% was obtained. The solution was placed in a three-neck flask, and 2g ZnCl2 was added and the mixture heated to 125-130°C. Through injection of steam into the reaction mixture the temperature was raised to 145-150°C, and a 50:50 solution of (-)-Pseudoephedrine freebase and 79% sulfuric acid (from an addition funnel heated to 70-90°C) was introduced into the reaction mixture at a rate of 1-5g/minute. The formed phenylacetone distills over with the steam, and is worked up as in Example 1. After 4h the reaction is stopped for 5 minutes, and thereafter the appropriate amount of sulfuric acid is drained from the reaction mixture to recreate the original volume. Thereafter the reaction is resumed. After removal of the formed methylamine hydrogen sulfate from the acid solution, it can be recycled into the reaction. The yield of phenylacetone is 81% of theory. Phenyl-2-Propanone from ephedrines via acid hydrolysis [2]The subject of the principal patent [1] is marked as a procedure for the production of Phenylacetone from Ephedrine by sulfuric acid (H2SO4), by the fact that one executes the conversion to P2P with 50-70 % acid at 150 to 155°C and in the presence of 0,02-0.5% ZnCl2 as a catalyst and that one continuously distillates the formed Phenylacetone out by means of steam distillation, directly from the beginning of the reaction. It was now found that one can execute the conversion also in the presence of 0,05 to 0.3 % of other metal chlorides in place of 0,02 to 0.5% zinc chloride. As other metal chlorides are suitable Iron(III)chloride and in particular aluminumchloride, borontrifluoride and Titan(III)chloride, which are used as Lewis acids in organic chemistry. The concentration of the sulfuric acid is selected in such a way, that it amounts to a content in the reaction mixture of 50-70 weight%. The reaction runs particularly well with a weight/weight(w/w) sulfuric acid content from approximately 60%. For the continuous discharge of the Phenylacetone from the reaction mixture, water vapour is initiated into the mixture, which removes the developing Phenylacetone immediately from the mixture. The received distillate is extracted with toluene and the latter is removed by distillation. Thus one obtains Phenylacetone, whith a purity of over 99,5%. For a source of Ephedrine comes Ephedrine, Pseudoephedrine, Norephedrine and Norpseudoephedrine as well as Bis-(1phenyl-2-methylaminopropyl1)-ether in consideration, whereby the reaction mechanism for such Ephedrines is particularly important, no direct results for so far for (-)-pseudoephedrin and (-)-norpseudoephedrin. The Ephedrine preferably is used in a weight/weight ratio of approximately 1:1 to 1:10, preferably 1:2 to 1:5 compared to the acid. The yields, which can be obtained with the new procedure, are within the 80% range. The procedure runs at a relatively high rate/min. A further advantage of the procedure consists of the fact that it can be executed continuously . Thus one can let a hot aqueous solution of the Ephedrine of choice flow into the hot acid, whereby the Phenylacetone/Water azeotrope continuously gets distillated out. Here it is only necessary, to remove the developing ammonium salt, e.g. methylammonium hydrogen sulfate occasionally. The use of the mentioned metal halides in place of ZnCl2 offers advantages regarding environmental protection, because these are more harmless and do not disturb the biological reduction of the waste water. Example 1 1025 g 75% sulfuric acid are mixed with 2 g AlCl3. In there one dissolves 426 g (2.58 mol) Ephedrine or Pseudo-ephedrine derivate from 50-100°C. Subsequently, the mixture is heated up to 145-150°C. At 125°C steam in moderate current is introduced in the fluid for better mixing. At 145°C one increases the steam introduction and distills in a period of 2 1/2 to 3 hours the Phenylacetone/Water azeotrope over. From the distillate one isolates by toluene extraction the raw Phenylacetone, which is free from Propiophenone. After distillation with a short Vigreux reflux column attached also, one receives Phenylacetone, yield 270 g (78%)with a purity upto 99.8 %. One gets similar yields from Nor-ephedrine or Nor-pseudo-ephedrine for Phenylacetone in a yield of approx. 80%. Example 2 In 1000 g 79% sulfuric acid, an amount of 100 g (-)-Pseudoephedrin-derivate gets dissolved, whereby the H2SO4-concentration is adjusted to 65-66 weight%. This solution is poured into one of the necks of a three-neck round-bottom flask, mixed with 4 g AlCl3 and heated up to 125-130°C. By injecting steam, one increases the temperature to 145-150°C and keeps it going in a steady rate from now on with a flow-rate of approximately 1 to 5 g/min. The 70-90°C warm solution of the (-)-pseudoephedrin(derivate) mixed with 79% sulfuric acid solution(weight ratio 1:1) is added by means of a steam-heated dropping funnel. The Phenylacetone turns thereby into an azeotrope and is similar to example 1 regenerated. In both cases, after 4 hrs, the reaction is interrupted for 5 minutes and during that 5 minutes there is so much sulfuric acid removed that the original volume is re-created. Then the reaction is continued. The removed sulfuric acid can be re-used after one removes the methylammoniumhydrogensulfat developed during the conversion. The yield of Phenylacetone amounts to 76%. Example 3 1350 g 79% sulfuric acid is added through a reaction flask neck and mixed with 590g 95% (+)-Ephedrine. 10 ml 18% TiCl3 is added subsequently, the solution mixed and the mixture heated to 125°C, and then heated to 145-155°C with steam, the distillate collected, from which by toluene extraction 358g Phenylacetone were isolated. From this, 335 g = 73.6 % yield of Phenylacetone, with a purity of 99.5% was collected by fractionated distillation over a Vigreux column. Example 4 In 1350 g 79% sulfuric acid is dissolved under agitating 561g dl-Ephedrine (99-100%), mixed with 15 g BF3/glacial acetic acid solution (10%) and heated to 125°C. One injects steam and increases the temperature thereby to 145-155°C whereby developed Phenylacetone is collected. After approx. 6 L steam distillate is collected, the conversion is terminated. One isolates 386.4 g Phenylacetone from the distillate by toluene extraction and distillation. From this, 367 g (80.6%) pure Phenylacetone is collected. Example 5 To 400 kg 60% sulfuric acid added in a distillation apparatus, 500 kg of 80% sulfuric acid are being gear-pumped. Added to this mixture is 400 kg (-)-pseudoephedrine (60 %) under mixing, and 1.5 L aluminum chloride solution (30 %) introduced. One heats the reaction mixture to 125-130°C and introduces then steam, while increasing the temperature to 145-150°C, whereby a mixture of steam/Phenylacetone distills over. After approx. 2000 L steam distillate is collected, one terminates distillation. From the distillate the lower heavy oil phase, consisting of Phenylacetone, is separated and the aqueous phase is extracted with approx. 400 L toluene. The separated Phenylacetone and the toluene extracts are combined and concentrated by distillation. The remaining arrears are afterwards fractionated distilled. One receives pure Phenylacetone to 155 kg (80%). References [1] German Patent 3,026,698
|