Ketamine is more difficult to synthesize than the previously considered PCP derivatives. Although it is currently a popular and common drug on the illicit market, it is obtained exclusively by diversion of commercial sources rather than synthesis. This route has an overall yield of ~60%, with a difficulty rating of 2-3 out of 10 and a hazard rating of 1-2 out of 10 (ref. 64). The general necessity of producing anhydrous methylamine in a clandestine setting, rather than purchasing it, increases the difficulty. Use of propylamine rather than methylamine would simplify this reaction, as its boiling point is above room temperature vs. methylamine, which is a gas at room temperature.
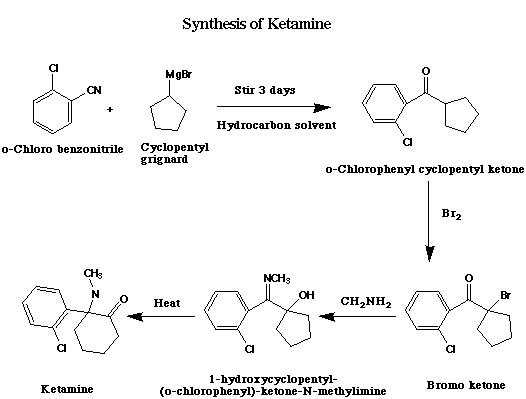
The syntheis starts with the reaction of cyclopentyl Grignard and o-chlorobenzonitrile to give o-chlorophenyl-cyclopentyl ketone, followed by alpha bromination of the ketone, and then reaction with methylamine to form an alpha-hydroxy imine (1-Hydroxycyclopentyl-(o-chlorophenyl)-ketone-N-methylimine). Heating this imine results in Ketamine via a novel alpha-hydroxyimine rearangement (refs. 20, 21, 22, 23, 24 ). Overall yields are ~60%.
Tiletamine is synthesized by an analogous process in industry, substituting 2-thiophenyl magnesium bromide for the phenyl grignard and ethylamine for methylamine. Two other ketamine analogs have been found on the black market: the compound missing the 2-chloro group on the phenyl ring, and its N-ethyl analog. Both of these compounds are most likely more potent and longer lasting than ketamine.

Step 1: (o-chlorophenyl)-cyclopentyl ketone
119.0 g of cyclopentyl bromide and 19.4 g of magnesium are reacted in ether or THF to give a cyclopentyl Grignard reagent. The best yields are obtained if the ether solvent is distilled from the Grignard under vacuum and replaced with hydrocarbon solvent, such as benzene. 55.2 g of o-chlorobenzonitrile is then added to the reaction mixture and stirred for three days. The reaction is then hydrolyzed by pouring it onto a mixture of crushed ice and ammonium chloride, containing some ammonium hydroxide. Extracion of the mixture with organic solvent gives o-chlorophenylcyclopentylketone, bp 96-97 C (0.3 mm Hg) (CAS# 6740-85-8).
Step 2: alpha-bromo (o-chlorophenyl)-cyclopentyl ketone
To 21.0 g of the above ketone is added 10.0 g of bromine in 80 ml of carbon tetrachloride dropwise at 0 deg. C. After all of the Br2 has been added, an orange suspension forms. This is washed with a dilute aqueous solution of sodium bisulfite and evaporated to give 1-bromocyclopentyl-(o-chlorophenyl)-ketone, bp 111-114 C (0.1 mm Hg). Yield is ~66%. This bromoketone is unstable and must be used immediately. Also attempts to distill it at 0.1 mm Hg lead to some decomposition, so it should be used without further purification.
The bromination may also be carried out with N-bromosuccinimide in somewhat higher yields (~77%).
Step 3: 1-hydroxycyclopentyl-(o-chlorophenyl)-ketone-N-methylimine
29.0g of above bromoketone is dissolved in 50 ml of liquid methylamine freebase. Benzene may also be used as solvent. After one hour, the excess liquid methylamine is allowed to evaporate, although increasing the reaction time to 4-5 days may increase yield. The residue is then dissolved in pentane and filtered. The solvent is evaporated to yield 1-hydroxy-cyclopentyl-(o-chlorophenyl)-ketone N-methylimine, mp 62 C (yield ~84%).
Step 4: 2-Methylamino-2-(o-chlorophenyl)-cyclohexanone (Ketamine)
The final step is a thermal rearrangement, and gives almost quantitative yield after 180 C for 30 min. An alternative to the use of decalin as solvent in this step is to use a pressure bomb.
2.0 g of the preceeding N-methylimine is dissolved in 15 ml of decalin and refluxed for 2.5 h. After evaporation of the solvent under reduced pressure, the residue is extracted with dilute hydrochloric acid, the solution treated with decolorizing charcoal, and the resulting acidic solution is made basic. The liberated product, 2-methylamino-2-(o-chlorophenyl)-cyclohexanone (Ketamine), after recrystallization from pentane-ether, has a mp of 92-93C. The hydrochloride has a mp of 262-263 C.
As with PCE, the freebase is too caustic to be smoked, and must be converted into the HCl salt in order to be consumed in this manner.