Phenethylamines from Benzyl Alcohols[ Back to the Chemistry Archive ] In view of the very broad pharmacological utility of substituted 2-phenylethylamines, we wish to contribute a synthetic procedure which, because of its versatility and convenience, may find considerable use. Although based entirely on standard synthetic methods, the overall scheme is specifically tailored to the properties of the benzylic intermediates involved, and eliminates the need for isolation of intermediates and other time-consuming operations. The procedure is described for the p-methoxy derivative; it is also applicable without substantive modification to other ring alkoxy-, alkyl-, and halogen-substituted phenethylamines. Procedure [1]p-Methoxy-phenylacetonitrile 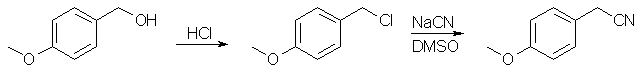
p-Anisyl alcohol (100 g, 0.725 mole) was shaken with 500 ml of concd HCl for 2 min. The organic phase was washed with H20, 5% NaHCO3 and H2O then added over 40 min to a stirred slurry of 49 g (1.0 mole) of NaCN in 400 ml of DMSO [2], with ice-water cooling to maintain the temp at 35-40°. After addition was complete, the cooling bath was removed, the mixture was stirred for 90 min and then added to 300 ml of H20, and the small upper phase separated. The aqueous DMSO layer was extracted with two 100-ml portions of Et2O which were combined with the product layer, and the whole was washed once with H20 and dried (MgSO4). p-Methoxy-phenylethylamine 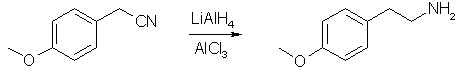
A dry flask was charged with ca. 600 ml of anhydrous Et2O and chilled in ice as 80 g (0.6 mole) of anhydrous AlCl3 was added portionwise, followed by 23 g (0.6 mole) of LAH (LAH alone and other metal hydride reagents are unsatisfactory for the reduction of benzylic nitriles to amines) [3]. The dried Et2O solution of crude p-methoxy-phenylacetonitrile was added at such a rate as to maintain gentle reflux without external heat (ca. 1 hr). The mixt was stirred for 2 hr, then chilled in ice, and treated dropwise with 25 ml of H2O followed by 250 ml of 20% of aq NaOH, with periodic addition of Et2O through the condenser to replenish losses and facilitate stirring. The resulting voluminous, granular ppt of NaCl and LiCl and aluminate was removed by filtration, washed well with Et2O and discarded. The filtrate was mixed with one-third its vol of abs EtOH and 60 ml of concd HCl was added slowly with continuous swirling and ice cooling. After chilling to 0°C, the crystalline amine hydrochloride was collected, 101 g, mp 212-214°C. The overall yield was 75% from anisyl alcohol. The hydrochloride may be recrystd from Et2O-EtOH or i-PrOH. References [1] Edgar F. Kiefer, J. Med. Chem. 15(2), 214 (1972).
|