Synthesis of Phenylpiperazines from Anilines1-(2,5-Dimethoxyphenyl)-piperazine Dihydrochloride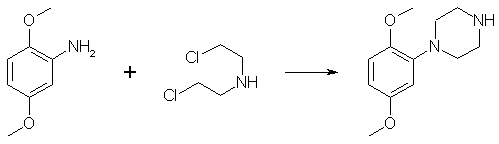
A mixture of bis(2-chloroethyl)amine hydrochloride (23.3g, 131 mmol), anhydrous K2CO3 (18g), freshly distilled 2,5-dimethoxyaniline (20.0g, 131 mmol) and diglyme (75 mL) was heated at reflux for 48 h, allowed to cool to room temperature, and then poured into water (200 mL). The aqueous mixture was made basic (about pH 12) by the addition of a saturated KOH solution and was extracted with ethyl acetate (3x200mL). The combined organic portions was washed with water (3x200mL), dried over MgSO4 and evaporated to dryness under reduced pressure to yield a dark oil. Vacuum distillation afforded 18 grams (62%) of the amine as a light-yellow liquid, bp 142-146°C at 0.18 mmHg. A saturated soln of HCl gas in anhydrous diethyl ether was added to a solution of the freebase in a small amount of 100% ethanol (can use methanol) to give the title compound, mp 218-220°C after recrystallization from 100% ethanol. 1-(4-Bromo-2,5-dimethoxyphenyl)-piperazine Dihydrobromide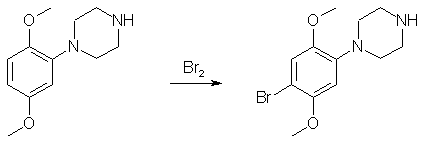
A solution of Br2 (3.2g, 20mmol)in glacial acetic acid (20 mL) was added in a dropwise manner to a solution of 1-(2,5-Dimethoxyphenyl)-piperazine freebase (4.0g, 20 mmol) in 48% HBr (4 mL) and glacial acetic acid (5 mL) at 0°C, and after the addition was complete, the solution was stirred at room temperature for another 4 h. The solvent was removed under reduced pressure to afford a white solid material (mp 220-224°C after recrystallization from 100% ethanol). This is the hydrobromide salt. Conversion of it to the hydrochloride is wasteful and may not be necessary. But if wanted, here goes: 1-(4-Bromo-2,5-dimethoxyphenyl)-piperazine Dihydrochloride2 grams of the above compound was dissolved in 10 mL H2O; the soln was made basic (to ca pH 9) with 10% aqueous NaOH and extracted with ethyl acetate (3x50mL), the combined organic portion was dried over MgSO4 and evaporated under reduced pressure to afford a dark oil. The oil can be distilled (optional step) with some difficulties, bp 72-75°C at 0.08 mmHg. The oil is then dissolved in 100% ethanol and dry HCl gas is bubbled through the solution until salt formation ceases. Recrystallization from 100% ethanol gave 0.3 grams of the title compound as small white crystals, mp 203-205°C (dec). Reference: J. Med. Chem. 29, 630 (1986) |