Benzaldehydes from Propenylbenzenesby Station[ Back to the Chemistry Archive ] IntroductionThe preparation of aromatic aldehydes by the oxidation of compounds with side-chains containing ethylenic linkages via potassium permanganate and potassium dichromate was investigated, including the oxidation of isosafrole to piperonal and of isoeugenol to vanillin. 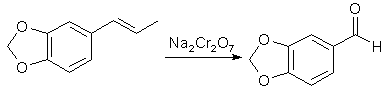
Since the initial materials are not completely miscible with water, the effect of introducing a dispersing agent was studied; for this purpose sulphanilic acid (which had the additional advantage of protecting the aldehyde when formed from further oxidation) and also Dispersol were used. The oxidation of isosafrole to piperonal by potassium permanganate under tested reaction conditions was far too vigorous, and the main product was piperonylic acid, whereas sodium dichromate and sulphuric acid afforded a 70% yield of piperonal, which was increased to 80% and 86.5% by the use of Dispersol and sulphanilic acid respectively as dispersing agents. The higher yield promoted by the sulphanilic acid was ascribed to ephemeral formation of a Schiff's base with the aldehyde when formed. With isoeugenol, in which the benzene structure is not immobilized by the clamping arrangement of the methylenedioxy group, the chromic acid oxidation gives a yield of 67% of vanillin as compared with the 86.5% of piperonal obtained from isosafrole. Experimental: The preparation of aldehydes(a) Without dispersing agent. A mixture of isosafrole (32.4g, 0.2 mol), 50% aqueous sulphuric acid (160 g) and water (1 L) at 30-40°C was vigorously stirred during the gradual addition over 30 minutes of a solution of sodium dichromate (44g, 0.13 mol, +10% excess) in water (200 ml). Reduction of the dichromate to green chromium salt appeared to be almost immediate; the mixture was twice extracted with benzene (600 ml), and the combined extracts were washed with 5% aqueous sodium hydroxide (200ml) followed by water (500ml). The extract was then dried over anhydrous calcium chloride, and the benzene removed, when the light brown residual oil crystallised on keeping (26g). The crude product was refluxed with ethyl alcohol (200 ml) and animal charcoal (10g), the crude solution filtered hot, and the bulk reduced to 100ml, when the piperonal was obtained in white crystals, mp 36-37°C (yield 21 g of pure product, 70%). (b) With sulphanilic acid as dispersing agent. Details were as in (a), except that the isosafrole was added to a solution of sulphanilic acid (12g) and sulphuric acid (80g, d 1.84) in water (1 L). Yield of pure product, 26 g. (86.5%). (c) Details were as in (a) except that Dispersol (5g) was present in the oxidation medium. Yield of pure product 24g (80%). Experimental--Variations in temperature and in amount of oxidizing agent used:
Experimental--Oxidation of isoeugenol to vanillin The best results were obtained by using the optimum conditions of the previous oxidation, viz., sodium dichromate (10% excess) with sulphanilic acid as dispersing agent. Yield, 67%. With alkaline potassium permanganate, only a 21% yield of vanillin was obtained. Experimental--The isomerization of safrole to isosafrole Metallic sodium (30 g.) was caused to react with ethyl alcohol (400 ml, dried over quicklime), and the mixture charged into a stainless steel autoclave (1 litre capacity) together with safrole (300 g.) and the temperature raised to 180°C during 2 hours, when the pressure was 300 lb. per sq. in. The temperature was maintained at 180-185°C for 6 hours; the contents of the autoclave were then poured into a solution of sodium chloride (200g) in water (1 L), and the oil was removed, washed with 20% sodium chloride (200 g.), and dried over anhydrous sodium sulphate. Two such preparations were combined and fractionated through a 2-ft. column packed with 1/4-in. Lessing rings; the results for the various fractions are given below.
Fraction 3 was employed for isosafrole in the oxidations above (literature gives d13 1.122; n20 1.5872). Reference: Davies and Hodgson, J.S.C.I., June 1943, 91-93. |