Synthesis of 4,5-Dimethoxy-2,3-Methylenedioxyallylbenzene (pseudo-Dillapiole)[ Back to the Chemistry Archive ] Procedure [1]3,4-Methylenedioxyphenol (Sesamol) 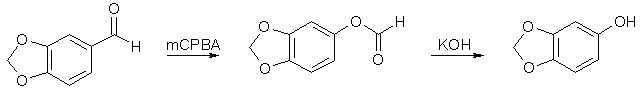
60g Piperonal was dissolved in 500ml DCM, and 90g MCPBA (m-Chloroperoxybenzoic acid) was added in portions, and the mixture stirred for 18h. The precipitate was filtered off and washed with DCM. The combined filtrates was then washed with cold saturated Na2CO3 solution, followed by H2O, dried over MgSO4 and evaporated to dryness. The resulting formate ester was dissolved in 250ml methanol and mixed with a solution of 30g KOH in 200ml methanol. The reaction mixture was stirred at room temp for 15h, evaporated to dryness, the residue dissolvd in 300ml water, neutralized with 6M HCl and extracted with ethyl acetate. The organic layer was washed with NaHCO3 followed by water, dried over MgSO4 and evaporated to dryness. The crude product (57g) was recrystallized from hexane to give colorless crystals of Sesamol (48g), mp 54-55°C. 3,4-Methylenedioxyanisole 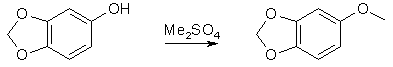
47g 3,4-Methylenedioxyphenol in 500ml dry acetone, 100g K2CO3 and 70ml dimethylsulfate was refluxed overnight. The white solid was filtered off, the filtrate concentrated, diluted with water and extracted with ethyl acetate. The organic layer was wahed with NaHCO3 solution and water, dried over MgSO4, and evaporated under vacuum to give 3,4-Methylenedioxyanisole. 6-Methoxy-2,3-Methylenedioxybenzaldehyde 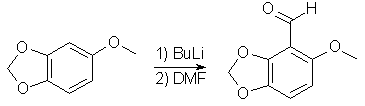
40g 3,4-Methylenedioxyanisole in 300ml absolute THF under argon at -10°C was stirred with 100ml butyl lithium in hexane (1.1 mol) for 2.5h. After the addition of butyl lithium the temperature was slowly raised to room temp. The aryl anion thus formed was stirred with 25ml dry DMF in 100ml THF at 0°C for 10 min, followed by refluxing for 2h. The reaction mixture was then acidified with 6M HCl and extracted with 1500ml ether. Drying over MgSO4 and evaporation of the solvent afforded a pale yellow crystalline compound, mp 124°C. 6-Methoxy-2,3-Methylenedioxyphenol 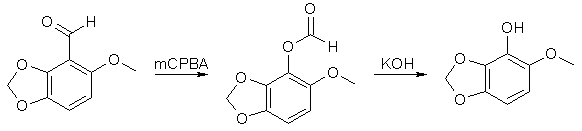
20g 6-Methoxy-2,3-Methylenedioxybenzaldehyde was stirred with 30g MCPBA (m-Chloroperoxybenzoic acid) in 500ml DCM for 18h. The precipitate was filtered off and the filtrate washed with Na2CO3 solution, and evaporated to dryness. The formate ester residue was stirred with 5% methanolic KOH for 2h, and resulted in 12g of a low-melting solid, which after recrystallization from hexane had mp 89-90°C. 1-Allyloxy-6-Methoxy-2,3-Methylenedioxybenzene 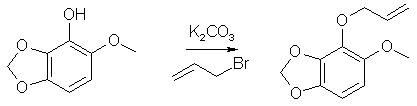
6g 6-Methoxy-2,3-Methylenedioxyphenol, 40g K2CO3 and 250ml dry acetone was stirred for 10 min, 8ml allylbromide added and refluxed for 18h. The resulting product gave 8g of 1-Allyloxy-6-Methoxy-2,3- Methylenedioxybenzene as a colorless oil after purification. 6-Methoxy-2,3-Methylenedioxy-4-allylphenol 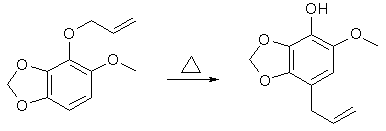
3.5g 1-Allyloxy-6-Methoxy-2,3-Methylenedioxybenzene was heated to 195°C under argon for 1.5h, cooled, dissolved in 200ml ether and extracted with 250ml 2N NaOH. The NaOH solution was backwashed once with ether, acidified with 6N HCl and extracted with 3x250ml ether. The ether layers was washed with Na2CO3, followed by water, and drying over MgSO4 and evaporated to dryness. The residue was recrystallized from hexane to give 2.7g of 6-Methoxy-2,3-Methylenedioxy-4-allylphenol, mp 52-53°C. 4,5-Dimethoxy-2,3-Methylenedioxyallylbenzene (pseudo-Dillapiole) 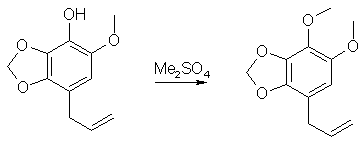
10g 6-Methoxy-2,3-Methylenedioxy-4-allylphenol was methylated as described above, and the product was obtained as a colorless liquid, yield 10g. References[1] US Pat 4,876,277 |