Abstract:
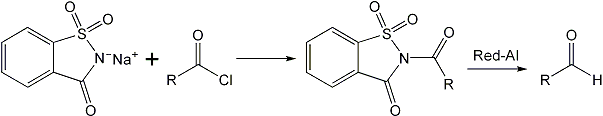
Synthesis of certain aldehydes from the carboxylic acids has been carried by reduction of the corresponding N-acyl saccharins using Red-Al. The N-acyl saccharins are easily prepared by reaction of an acid with y-saccharin chloride, which can be used for the next step of reduction without further isolation and purification. This provides a convenient and one step synthesis of aldehydes by combining the two reactions, viz, preparation of the N-acyl compound and its reduction. The method has been successfully applied for the reduction of aliphatic, alicyclic, aromatic and alpha, beta- unsaturated acids to the corresponding aldehydes and the yields obtained are quite satisfactory.
Explanation of the Method:
Numerous routes to aldehydes from carboxylic acid derivatives are known1,2. The recent methods of aldehyde synthesis from acids or their derivatives utilize metal hydride reduction. Normally, the metal hydride reduction of acid derivatives, e.g., the esters or the acid chlorides gives the corresponding alcohols while the amide derivatives are reduced to amines or alcohols, but during the reduction of tertiary amides and in case of certain phenolic esters, the rupture of the amide bond or ester bond has frequently been observed, which results in N- or O- deacetylation, respectively. If the hydride is not used in excess, the acyl function can be isolated as the corresponding aldehyde, particularly if the reduction is carried out at a low temperature. A comprehensive survey of such methods of aldehyde synthesis has recently been made by Smith3 and Weygand4-6.
In many of these methods, the N-acyl compound is derived from more acidic –NH- groups such as those in carbazole7, 3,5-dimethylpyrazole8,9, imidazole and pyrrole. This is to be expected because such N-acyl compounds are active trans-acylating reagents and the amine component in such compounds can act as a good leaving group, resulting in easy cleavage or –CO-N—bond. The same is true of phenolic esters.
| # | N-acyl saccharin | Aldehyde | bp°C | Yield |
| 1 | N-acetyl- | acetaldehyde | 18°C (20) | 76% |
| 2 | N-propyl- | propionaldehyde | 53°C (49) | 78% |
| 3 | N-butyryl- | butyraldehyde | 72°C (75) | 78% |
| 4 | N-cyclohexanecarbonyl- | cyclohexanealdehyde | 66°C (63) | 75% |
| 5 | N-benzoyl- | benzaldehyde | 182°C (179) | 80% |
| 6 | N-(o-chlorobenzoyl)- | o-chlorobenzaldehyde | 210°C (213) | 65% |
| 7 | N-(p-chlorobenzoyl- | p-chlorobenzaldehyde | 212°C (214) | 72% |
| 8 | N-(o-tolyl)- | o-tolualdehyde | 201°C (200) | 63% |
| 9 | N-(p-toluoyl)- | p-tolualdehyde | 206°C (204) | 70% |
| 10 | N-(p-nitrobenzoyl)- | p-nitrobenzaldehyde | 103°C* (106) | 75% |
| 11 | N-cinnamoyl- | cinnamaldehyde | 253°C (252) | 77% |
* MP of p-nitrobenzaldehyde
An aldehyde synthesis introduced by Staab10, involves the reduction of N-acylimidazole. Recently, the trans-acylation reactions of N- acylsaccharin were reported from these laboratories11 and the reactivity of N-acyl saccharin has been found to be comparable to that of N- acyl imidazole. It has now been found that the N-acyl saccharin can be synthesized in one-step from the carboxylic acid and y-saccharin chloride which is easily prepared9. N- acyl saccharins thus synthesized can be reduced in high yields to the corresponding aldehydes by sodium bis-(2- methoxyethoxy)-aluminum hydride (Red-Al). This provides a convenient and one-step synthesis of aldehyde from the carboxylic acid. The main advantage of the proposed method is combining of the two reactions, i.e., the preparation of N-acyl compound and its reduction in one step. The reductions are successfully carried out at 0-5°C, whereas in many cases, temperatures as low as –70°C have to be employed. The method has been successfully applied for the reduction of aliphatic, alicyclic, aromatic, and alpha, beta-unsaturated acids to the corresponding aldehydes.
Experimental:
Preparation of N-Acyl Saccharins
From the Acid Chloride
A mix of the acid chloride (1 mol) and sodium salt of saccharin (1.5mol) was melted together for a few minutes. The products obtained as such were washed with NaHCO3 and finally with recrystallized from ethyl methyl ketone or THF.
From y-Saccharin Chloride
To a solution of y-saccharin chloride (1mol) in DCM was added the acid (1 mol) at 0°C12. The mix was kept at this temperature for 10 min or such time till the completion of the reaction which was analyzed by TLC. Finally, the DCM was distilled off and the N-acyl saccharin obtained was subjected to reduction.
Reduction of N-Acyl Saccharins by Red-Al
To a suspension of the N-acyl saccharin (1mol) in dry benzene, was added with stirring, a solution of Red-Al (0.5mol) in benzene, in small portions, during 5-10 min at about 0-5°C. The stirring was continued at the temperature for about 2 h. The product was subsequently decomposed with little water. The benzene layer was separated and the precipitate of saccharin and the metal hydroxide was filtered off and washed with benzene.
The aqueous layer was extracted several times with benzene and all the benzene extractions were combined and subjected to reduced pressure distillation using rotary vacuum flash evaporator. In this manner, the aldehydes were found to be in the range of 70-80%. Alternatively, the combined benzene solution may be treated with a solution of 2,4-dinitrophenyl hydrazine and the precipitated hydrazones can be weighed. The results obtained by the reduction of various N-acyl saccharins with Red-Al are given in Table.
Saccharin is itself not reduced by Red-Al.