Both 3,4,5-trimethoxyamphetamine (I, TMA)2 and 3-methoxy-4,5-methylenedioxyamphetamine (II, MMDA)3 have exhibited psychotropic potencies greater than that of mescaline. Both the lengthening of the aliphatic chain of I4 and the enlargement of the heterocyclic ring in II3 have resulted in a decreased human effectiveness. On the contrary, it has been found that the repositioning of the meta-methoxy group, in either of these compounds, to an available ortho-location, can result in an appreciable increase in potency. The syntheses and preliminary pharmacological evaluation of these positional isomers are described.
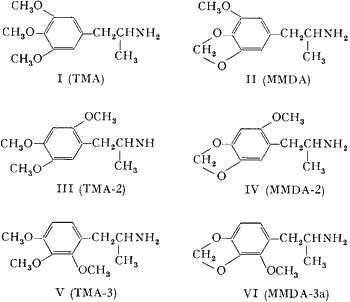
Two methods of synthesis were employed. With the two bases possessing the 2,4,5-alkoxy orientation, the appropriately substituted propenylbenzene was oxidized with tetranitromethane to the corresponding β-nitropropene. LiAlH4 reduction of these nitrostyrenes to the amphetamines was performed as previously described5. The propene required for III, asarone, was obtained by the fractional distillation of oil of Parsley. The necessary precursor of IV, 2-methoxy-4,5-methylenedioxy propenylbenzene was obtained by conventional steps from sesamol. The Claisen rearrangement of allyl sesamyl ether occurred predominantly to the unhindered side; O-methylation and base-catalyzed isomerization yielded the requisite propenylbenzene.
The vicinal analogs V and VI were both prepared from the corresponding benzaldehydes by condensation with nitroethane followed by reduction of the resulting nitrostyrene as described above. The properties of these products, together with those of the 3,4,5-analogs for comparison, are listed in the Table.
Physical and pharmacological properties
| Compound | Nitrostyrene mp | Base•HCl mp | EDa (mice) | LD50 (mice) | EDb (human) | M.U.c |
| II) TMAd | 94°C |
209°C |
20 mg/kg |
260 mg/kg |
1700 µg/kg |
2.2 |
| II) MMDA | 110°C |
191°C |
35 mg/kg |
150 mg/kg |
1400 µg/kg |
2.7 |
| III) TMA-2e | 102°C |
181°C |
15 mg/kg |
120 mg/kg |
220 µg/kg |
17.0 |
| IV) MMDA-2 | 163°C |
187°C |
20 mg/kg |
130 mg/kg |
180 µg/kg |
21.0 |
| V) TMA-3 | 57°C |
149°C |
35 mg/kg |
120 mg/kg |
>1900 µg/kg |
<2.0 |
| VI) MMDA-3a | 106°C |
154°C |
25 mg/kg |
40 mg/kg |
210 µg/kg |
18.0 |
- Effective dosage (as free base in saline, i.p.) at which behavioral changes were observed.
- Effective dosage (as free base, per os) defined as the arithmetical mean between the
minimum detectable dosage and the dosage above which there is a prolongation rather
than an intensification of the psychotomimetic syndrome. - Mescaline Units (human). The quotient of the effective dosage of mescaline (assumed to
be 3750 µg/kg as the base) divided by the effective dosage of the substance in question.
It permits a direct comparison of relative potencies, based on mescaline = 1. - Physical data from 4.
- Literature values for the nitrostyrene mp 101°C and for the base•HCl, 187°C, see
V. Bruckner, J. Prakt. Chem. 138, 268 (1933)
Behavioral and toxicological studies were performed on male Swiss white mice, and these results are also recorded in the Table. All four isomers were found to be somewhat more toxic than the 3,4,5-substituted analogs. Compound VI, MMDA-3a, was the most toxic of the group and it alone produced clonic convulsions and vocalization prior to death. The other compounds led to easy deaths, apparently due to respiratory paralysis. All compounds displayed initial behavioral changes at the levels listed. The responses observed were light tremors accompanied by rapid scratching and a huddling tendency. These effects disappeared within 3 h, and there were no noticeable after-effects.
The intoxication syndrome in human subjects, resulting from the quantities shown in the Table, is qualitatively similar to that which results from mescaline, except that the color effects, and to a large extent the nausea, are absent. As a generalization, the MMDA series leads to the more empathic and pleasant responses, whereas personal anxiety and restlessness were common with TMA-2. The vicinal analog, TMA-3, demonstrated neither physical nor psychotropic effects, even in dosages in excess of those shown to be adequate for TMA and MMDA. With the other three ortho-methoxy derivatives, however, hypnogogic hallucinatory synthesis and total recall are present and are similar to mescaline.