Phenylnitropropene Reduction to the P2P Oximeby Barium[ Back to the Chemistry Archive ] 1-(2,4,5-trimethoxyphenyl)-2-propanone oxime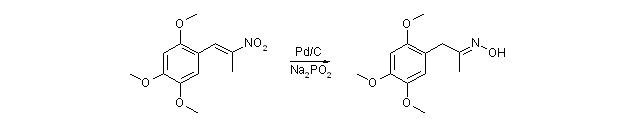
25,3 g (100mmol) 1-(2,4,5-trimethoxyphenyl)-2-nitropropene was suspended in 100ml IPA or EtOH and 800mg 5% Pd/C was wetted with IPA and added to the suspension. 39g (370mmol) sodium hypophosphite monohydrate was dissolved in 50ml warm water and added in portions to the nitroalkene suspension under violent stirring. The exotherm will easily bring this to a boil, but keep it at 40-50 deg C by immersion in a cold water bath. The reaction is finished when the top alcohol layer is colorless or has just a very slight yellow color left, this takes roughly two hours. Celite was added (three or so teaspoons) and the mixture is stirred for a minute and then filtered at the water pump. The filtrate quickly separates in two layers. The bottom aqueous layer was removed and the top layer was transferred to a rotovap, and the alcohol removed by distillation. This leaves a oil which quickly forms crystals which can either be dissolved in some DCM or toulene, filtered from a small amount of salts and stripped of solvent to give 23,4g (98%) 1-(2,4,5-trimethoxyphenyl)-2-propanone oxime, purity 98%. The oxime can then be further reduced to the amine (TMA-2), or the crude oxime can be hydrolysed to the ketone by acid hydrolysis. I have performed this reaction for about 6 months now. In the beginning I only got some 70% yield. But now, without having really changed anything, I get 97-98% everytime. Wierd! When using the nitropropenes from piperonal or benzaldehyde as substrates in the CTH method used to make 2,4,5-triMeOP-2-P oxime, the result is a little diffrent.
Low temp and high catalyst loading gives more oxime and high temp (reflux), more water, addition of acetic acid and low catalyst loading gives more ketone. The ketones are separated from the oximes easiest by steam distillation. EtOAc can also be used as solvent in the CTH. This gives better yields of oximes which are sensitive towards hydrolysis. Such a reaction would look like this:
If substrate, catalyst, aqueous hypophosphite, EtOAc is combined in a flask nothing much happens until 4-5ml IPA or EtOH is added. A nice way to avoid ignition of the solvent is to add the catalyst first to the reaction flask, then the dry substrate. Make sure the substrate really covers the catalyst. Then add the EtOAc followed by the alcohol, and finally aq. hypophosphite in portions. |