Tryptamine is a very important compound not only because of its use as a starting material in the syntheses of indole alkaloids, but also because of its pharmacologic activity. Since it is also very expensive, there are many reports on the syntheses of tryptamine2,3.
Although we had already reported a one-step synthesis of tryptamine from L-tryptophan4, the solvent, diphenylmethane, used in this reaction was expensive. In order to solve this problem, we also investigated another synthesis of tryptamine. Here we wish to report a convenient and inexpensive two-step preparation of tryptamine from L-tryptophan.
It is well known that an amino acid forms a chelate compound with metal5 and that a metal ion facilitates the decarboxylation of oxocarboxylic acids6.
On the basis of the above facts, we have investigated the decarboxylation of metal chelate compounds (1) and (2), prepared from L-tryptophan with copper(II)acetate and zinc acetate7. Thus, in case of copper(I)chelate compound (1), decarboxylation proceeded readily in aprotic solvents such as hexamethylphosphoric triamide (HMPA) and dimethyl sulfoxide (DMSO), but not in protic solvents such as water and 10% hydrochloric acid. Reactions of 2 in hexamethylphosphoric triamide, dimethyl sulfoxide, and water gave mainly tryptophan.
It is clear that a formation of tryptamine would be accelerated by an inductive effect due to the co-ordinated metal ion.
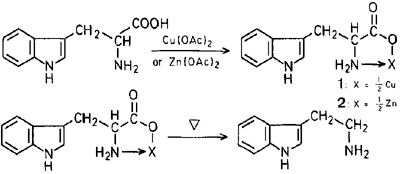
Conversion of 1 to Tryptamine
| Solvent | Temp. | Time | Yield |
| HMPA | 170-175°C |
3 min |
45% |
| DMSO | 170-175°C |
10 min |
40% |
| (CH2OH)2 | 190-200°C |
20 min |
10% |
| Phenol | 180°C |
60 min |
5% |
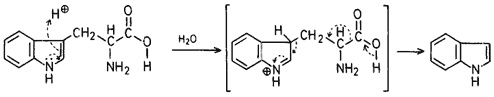
The formation of indole as by-product could be explained by the following mechanism.
Experimental
Copper Chelate Compound (1)
To a solution of L-tryptophan (50 g) in water was added a solution of an excess of copper(II)acetate in water. The resultant precipitate was filtered. The extract was then washed several times with hot water to give the copper chelate compound. Yield: 52 g, mp >280°C.
Zinc Chelate Compound (2)
An excess of zinc acetate in water was added to a solution of L-tryptophan (50g) in water. The resulting mixture was then heated for 10 minutes on a water-bath. The resultant precipitate was filtered and washed several times with hot water to afford the zinc chelate compound. Yield: 45 g, mp >280°C.
General Procedure
A suspension of metal chelate compound (1 or 2), prepared from L-tryptophan as above with copper(II)acetate or zinc acetate, in a variety of solvents was heated at 170-175°C for several minutes, during which time an evolution of carbon dioxide was observed.
After cooling, the resultant precipitate was filtered and to the filtrate was added a suitable amount of water. The reaction mixture was made basic with 30% sodium hydroxide solution and extracted with chloroform. After distillation of the solvent, the resultant residue was purified by chromatography on silica gel. When hexamethylphosphoric triamide and dimethyl sulfoxide were used as solvents, tryptamine was produced in 40-45% yield. The table records the results of the above reactions.
When water was used as solvent, the reaction was carried out in an autoclave. Some indole was formed and separated by steam distillation and subsequent extraction with chloroforms. In each case. Tryptamine was identified as its hydrochloride, mp 246-248 (Ref.4 mp 248-249°C).