by Erowid
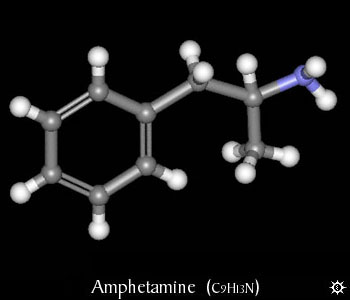
| NAME : | Amphetamine |
| CHEMICAL NAME : | (±)-alpha-Methylbenzeneethanamine |
| ALTERNATE CHEMICAL NAMES : | dl-alpha-methylphenethylamine, 1-phenyl-2-aminopropane, beta-aminopropylbenzene |
| ALTERNATE CHEMICAL NAMES : | racemic desoxy-nor-ephedrine, Actedron, Allodene, Adipan, Sympatedrine |
| ALTERNATE CHEMICAL NAMES : | Psychedrine, Isomyn, Isoamyne, Mecodrin, Norephedrane, Novydrine, Elastonon |
| ALTERNATE CHEMICAL NAMES : | Ortedrine, Phenedrine, Profamina, Propisamine, Sympamine, Simpatedrin |
| CHEMICAL FORMULA | C9H13N |
| MOLECULAR WEIGHT | 135.21 |
| From the Merck Index 12th Edition | |
|---|---|
MERCK INDEX ENTRY
623. Amphetamine. (±)-alpha-Methylbenzenethanamine; dl-alpha-methylphenethylamine; 1-phenyl-2-aminopropane; (phenylisopropyl)amine; ß-aminopropylbenzene; racemic desoxy-nor-ephedrine; Acetedron; Allodene; Adipan; Sympatedrine; Psychedrine; Isomyn; Isoamyne; Mecodrin; Norephadrane; Novydrine; Elastonon; Ortedrine; Phenedrine; Profamina; Propisamine; Sympamine; Simpatedrin. C9H13N; mol wt 135.21. C 79.95%, H 9.69%, N 10.36%, Prepn: U.S. pats. 1,879,003 (1932); 1,921,424 (1933); 2,015,408 (1935); Hartung, Munch, J. Am. Chem. Soc. 53, 1875 (1931). Demonstration of sterospecific binding sites for (+)-³H-amphetamine in hypothalamic membranes and correlations with anorexic potency of phenylethylamines: S. M. Paul et al., Science 218, 487 (1982). Toxicity data: M.R. Warren, H.W. Werner, J. Pharmacol. Exp. Ther. 85, 119 (1945); W.A. Behrendt, R. Deininger, Arzneimittel-Forsch. 13, 711 (1963). Series of articles on the biochemical and behavioral effects of amphetamines in man and animals: Handb. Exp. Pharmakol. 45, 3-304 (1977); Handb. Psycho pharmacol. 11, 1-98 (1978). Review of use and abuse: J. P. Morgan, Substance Abuse: Clinical Problems and Perspectives, J. H. Lowinson, P. Ruiz, Eds. (Williams C Wilkins, Baltimore, 1981) pp 167-184. Books: C. D. Leake, The Amphetamines: Their Actions and Uses (Thomas, Spring field, 1958) 167 pp; O. J. Kalant, The Amphetamines: Toxicity and Addiction (Thomas, Springfield, 1966) 151 pp.
Mobile liquid. Amine odor. Acrid, burning taste. Vola tilizes slowly at room temp, d25/4 0.913. bp760, 200-203°; bp13 82-85°. Slightly soluble in water; sol in alc, ether; readily sol in acids. Aq solns are alkaline to litmus. LD50 in rats (mg/kg): 180 s.c. (Warren, Werner).
Sulfate, (C9H13N)2.H2SO4, Alentol, Benzedrine, Psychoton, Simpamina. Crystals. Slightly bitter taste followed by sensation of numbness. mp above 300° (dec). One part dissolves in 8.8 parts water, 515 parts 95% ale. A soln of 1 g/10 ml water has a pH 5-6. LD50 in mice, rats (mg/kg): 24.2, 55 orally (Behrendt, Deininger).
Phosphate, C9H13N.H3PO4, Actemin, Aktedron, Monophos, Profetamine Phosphate, Racephen, Raphatamine Phosphate. Prepn: Goggin, U.S. pat. 2,507,468 (1950 to Clark & Clark). Crystals, bitter taste. Sinters at about 150°. Dec around 300°. More sol in water than amphetamine sulfate. Slightly sol in alcohol. Practically insol in benzene, chloroform, ether. The pH of a 10% soln is about 4.6.
d-Form tannate, tanphetamin, Synatan. Prepn: Cavallito, U.S. pat. 2,950,309 (1960 to Irwin, Neisler and Co.).
d-Form sulfate, see Dextroamphetamine Sulfate.
l-Form, levamphetamine, levamfetamine.
l-Form succinate, Cydril.
Note: This is a controlled substance (stimulant) listed in the U.S. Code of Federal Regulations, Title 21 Part 1308.12 (1995). THERAP CAT: CNS stimulant; anorexic. THERAP CAT (VET): CNS stimulant, in narcotic poisoning anesthetic collapse, in depression from encephalitis.


