LSD Analysis
Do we know what's in street acid?
Nov 2003
Citation: Erowid F, Erowid E. "LSD Analysis". Erowid Extracts. Nov 2003;5:12-17.
According to the 2002 National Household Survey--the largest survey of psychoactive drug use in the United States--more than 10% of people over the age of 12 say they have tried LSD at some time in their lives. The number is more than 15% among those between the ages of 18 and 25.1
Although it has now been over 60 years since the discovery of LSD--with thousands of papers published about its chemistry, pharmacology, and social impact--there are still huge questions about what is sold on the street as "acid".
Some people believe that there is no pure acid on the street these days: that the last "clean" acid was made by Sandoz or Owsley.2 Others believe that "acid is acid": that differences in purity don't play a significant role in different effects, which are instead explained as the result of differences in dosage, set, setting, and individual reactions. Yet a third opinion is that there are variations in purity--from incomplete syntheses, poorly purified material or degraded material--as well as a variety of chemicals such as ALD-52 or isomers of LSD that might be sold as street acid.
One of the primary arguments against the premise that differences in LSD experiences are the result of differences in quality of material has come from people we've spoken to who have distributed and aliquoted acid in the past. One such person described how some recipients of his LSD would go on at length about how distinct and how much better one type of blotter was than another. Yet, often, both types had been aliquoted by this chemist on the same day, from the same batch of liquid, onto similar blotter paper bearing different designs.
Unfortunately, getting street LSD legally analyzed is extremely difficult for anyone outside of law enforcement. Starting in the early 1970s with the passage of the Controlled Substances Act, tight restrictions were put in place over who is allowed to analyze and research controlled substances. Only those labs or individuals who are licensed by the DEA are allowed to handle Schedule I substances such as LSD.
In 1972, the BNDD (The Bureau of Narcotics and Dangerous Drugs) requested that labs collect names and addresses for anyone submitting controlled substances for testing.3 Then in 1974, the DEA issued firm guidelines that required licensed laboratories to stop providing quantitative results for anonymously submitted samples of scheduled substances.4 Now, unless a person is willing to walk in to a lab, give their name, show identification (which would be recorded in the lab's records), and then submit the sample (thereby volunteering proof of having committed a crime), labs are not permitted to provide information about how much of any chemical is present in the sample. Not surprisingly, most lawyers would advise against such an action.
Additionally, even for qualitative data (simply listing what chemicals are present) the DEA does not allow labs to test anonymously submitted samples without special permission. It is through one such approved lab that the EcstasyData project is, with some difficulty, able to provide qualitative results for street ecstasy tablets.
Though these rules against testing are only DEA "guidelines" (we do not believe they are part of any law or publicly available official policy), representatives of at least one lab we spoke with have been threatened with having their license revoked after participating in research the DEA didn't like. Since DEA licensing is required in order to handle Schedule I substances, labs that work with such chemicals are understandably reticent to risk their livelihood by going against these guidelines.
Unfortunately, because of the combination of DEA restrictions and the difficulty of testing, there may be no labs in the United States currently licensed and qualified to do anonymous testing of LSD.
Even here, the resources are scarce. To the best of our knowledge, there have been no papers published, using reproducible lab methodology, that attempt to characterize the impurities found in street acid.
Prior to 1974, PharmChem published both qualitative and some quantitative testing results--including microgram dosages for street LSD--in their print newsletter. Each month they would publish a list of hundreds of samples that had been submitted, including information about what the submitter believed the substance to be, where and when it was purchased, how much it had cost, a physical description of the material, and what the test results were for the sample. Unfortunately, they failed to describe their testing methods. We believe, based on talking to Alexander Shulgin and a former PharmChem employee who worked there after their "Analysis Anonymous" program had ended, that they used thin layer chromatography to detect both the presence of LSD, and what could only be estimates of the quantities present.
In March 1977, PharmChem published a review of the street drug analysis they had done in the United States and Canada between 1969 and 1975. Looking at a total of 2200 samples submitted as "acid" or "LSD", they reported that 87% of the submitted samples alleged to be LSD were d-LSD with no other psychoactive drug present. In LSD-positive samples, they found a range of 5 to 500 µg with an average of 75 µg per dose.5
Interestingly, people who submitted samples to PharmChem also often submitted comments about whether they believed that the acid contained "speed" or "strychnine". Although no strychnine was ever detected in any of the submitted samples--and only a few tested positive for methamphetamine--PharmChem reported that the more LSD that was present in a dose unit, the more likely the submitter was to think it contained strychnine.
Following the issuance of the DEA's 1974 guidelines, PharmChem and other labs quickly stopped publishing quantitative data.
According to the DEA, the average dose of LSD on street blotter is between 20 and 80 micrograms (µg). Based on this, they now consider a "standard dose" of LSD to be around 50 µg. They also report that crystal LSD they have seized averages about 62% d-LSD (with the rest made up of other isomers or impurities) but that some is 95-100% pure.6
For more than 35 years, the DEA and their predecessor organizations have produced a private--in fact almost secret--internal publication called Microgram. Prior to 2003, this publication was available only to the DEA and the labs and chemists they worked with. A wide variety of information about the street use of scheduled substances and about law enforcement action has been published in Microgram. Over the years, it has included information about LSD blotter designs tested by DEA offices around the United States and the lab techniques used to test them. They have also regularly printed images of street acid and sometimes included quantitative information.
In 2002, in response to Erowid's interest in learning more about the DEA's past LSD analysis, a Freedom of Information request was filed for a 10-year-old issue of Microgram. The request was denied and is currently being appealed.
Still seeking the information, we were soon informed that some obscure libraries in the United States happen to carry back issues. From these libraries and from Erowid friends, we have been able to piece together a collection of back issues of the publication.
The latest year of Microgram we have access to is 1996. In that year, the DEA published a handful of blotter images and a small number of quantitative test results. The doses they report were between 28 and 78 micrograms per hit of blotter.
More interestingly, in 1987, the DEA published an article in Microgram titled "The LSD Blotter Index" by Franzosa, Harper, and Crockett. This article is a catalog of hundreds of blotter designs seized by law enforcement between 1976 and 1986, including quantitative information for many of them.7 In this index, the reported dose per unit ranges from as low as a couple of micrograms to doses near 200 µg per hit.
Unfortunately, Microgram is not scientifically rigorous in its reporting of the DEA's testing results. No information is provided in The Blotter Index about which labs provided which data and what methods were used for detection or quantification. Likewise, the DEA's data about average doses is difficult to reproduce or verify, as their methods for averaging are not described and they do not provide information about how many doses of each design were produced, seized, or tested.
The dose per unit for individual designs described in The Blotter Index also shows an average of about 20-80 µg per hit. There is speculation that some of this variation might be the result of inconsistency in the dipping process resulting in higher doses on one edge of a sheet than the other. A copy of this article is available in the Erowid LSD Vault.
The Netherlands have an ongoing free testing program where individuals can bring in a sample of any street drug to be tested. We were given a summary of their testing results that shows that they receive very few samples of street acid to be tested. Of 600 samples submitted in 2001 and 2002, they tested only 12 samples alleged to be LSD and only half of these actually contained LSD. Of these, they reported doses ranging from 5 to 168 micrograms. Additionally, the person we communicated with from their lab said that results for the two lowest dose samples were considered unreliable. The Dutch lab reports that they use HPLC for their quantification and occasionally use GC/MS to verify results.8
Although we have been unable to get results from or clear documentation about ongoing testing programs in other countries, we believe that testing programs for street drugs exist in France, Spain, Austria, and Switzerland, most funded by the national governments. Most of these programs are oriented towards testing ecstasy tablets, cocaine, or heroin and it is unclear whether they could reliably detect or quantify various forms of LSD, let alone detect minor impurities.
Information about these brown microdots was reported to Erowid from around the United States between August 2002 and May 2003. Additionally, the newly public version of the DEA's Microgram reported a seizure of two such brown microdots in Owatonna, Minnesota in April 2003.9 Though the Microgram mention did not include quantitative information, most reports (though not all) have described these microdots as "very weak", suggesting a low dosage per tablet.
The lab we communicated with used high performance liquid chromatography (HPLC), ultraviolet (UV) absorption measurements, and liquid chromatography/mass spectrometry (LC/MS) to determine what was in the microdot. Using the same methods, the microdot results [Fig. 1] were compared against both the high quality analytical standard [Fig. 2] and the 10-year-old lab sample [Fig. 4].
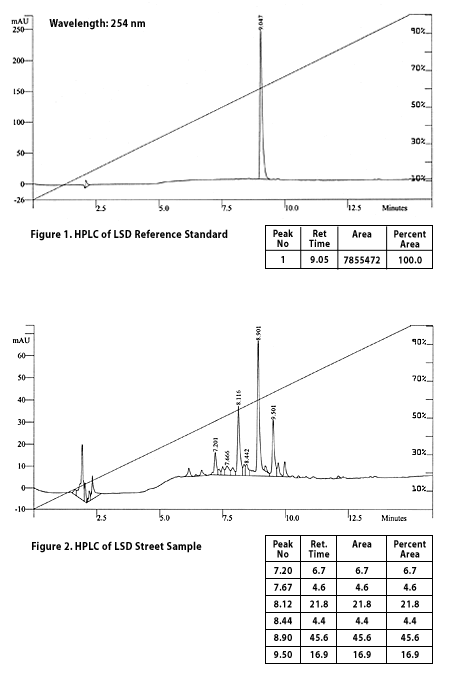
At the end of the column is a detector that senses when the chemicals emerge from the tube. The output from the column is subjected to ultraviolet light at a chosen wavelength. For this test 254 nanometer light was used, a common detection frequency.
The time the sample takes to move through the column is called the "retention time" and is marked along the x-axis (bottom side) of figures 1, 2, and 4.
The y-axis (left side) of the charts shows how much UV light was absorbed by the material coming out of the column at a particular point in time.
Each peak in the chart represents when each chemical component of a sample reached the end of the column. The small bumps nearest the left side of each chart are called "solvent fronts" and are usually ignored because they represent residue chemicals carried through the system with the initial "wave" of the injected solvent.
The HPLC + UV output for the new reference standard of d-LSD (Fig. 1) is quite simple. In the middle of this figure, there is a single very clean, clear peak with a retention time of 9.047 minutes. Using an electro-spray mass spectrometer, the lab verified that this reference standard had the correct molecular weight (323) for d-LSD. This material was further verified by checking that its UV absorption profile had the correct peak absorption at around 320 nanometers.
This all verified that the new reference standard appears to be very pure d-LSD. Although there are some extremely minor perturbations in the baseline around the LSD peak, these are well within the margin of error of the equipment.
Since each peak represents a chemical present in the sample, and there are several significant peaks besides the one known to be d-LSD, that leaves us with the important question--what chemicals do the rest of the peaks represent? Relative amounts of each chemical can be measured by comparing what is known as Area Under the Curve (AUC), shown in the table below the graph.
In figure 2, peak 3 (at 8.12 minutes) is nearly half the area of the d-LSD peak and peak 6 (at 9.5 minutes) is a little over a third of the area of the d-LSD peak. This suggests that the chemicals represented by those peaks are present in the microdot sample in quantities one-half and one-third of the total d-LSD present, respectively. Because the exact retention times for specific substances can vary quite a bit, based on factors such as what type of solvent and column were used, retention time cannot be used by itself to identify a substance.
 We believe that peak 6 is iso-LSD, an isomer of LSD. The lab guessed this peak to be iso-LSD based both on its UV absorption profile (image not shown) and on their analysis of results of their LC/MS work (also not shown).
We believe that peak 6 is iso-LSD, an isomer of LSD. The lab guessed this peak to be iso-LSD based both on its UV absorption profile (image not shown) and on their analysis of results of their LC/MS work (also not shown).
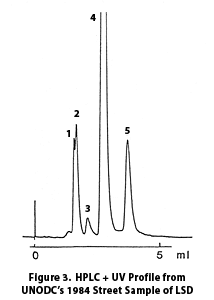
This is also confirmed by a 1984 article published by the United Nations Office on Drugs and Crime,10 which discussed analytical work done in the United Kingdom. Figure 3 is an image from this paper showing the HPLC + UV profile for a street sample tested in 1984. The similarities to the results from the brown microdot are striking. This paper identifies peak 4 (the equivalent to peak 5 in the microdot sample) as d-LSD, peak 5 (equivalent to microdot peak 6) as iso-LSD, peak 3 (equivalent to microdot peak 4) as most likely unconverted ergotamine, and says peaks 1 and 2 are unidentified.
Iso-LSD is considered non-psychoactive. Papers in the late 1950s reported low or no activity for iso-LSD, including a very interesting report published by Sandoz comparing activities of several "lysergic acid derivatives".11 Albert Hofmann reported having tried iso-LSD at doses up to 500 µg and found no psychoactivity. More recent rat experiments have found that rats given iso-LSD in a discrimination study don't respond as though it's d-LSD.12 It is an open question, however, whether high levels of iso-LSD could possibly alter the effects of LSD, either through activity at a different serotonin receptor type than d-LSD triggers or through some other mechanism.
As far as we know, no research has been done to determine whether isomers of LSD can potentially reduce or alter the receptor binding or otherwise modify the pharmacodynamics of d-LSD. At this point, iso-LSD is mostly believed to be inert and simply represents "wasted" lysergic material since it is in the wrong configuration to be psychoactive.
The rest of the small peaks in that area of the graph are likely other lysergic-structure chemicals, although without doing work to characterize exactly what those peaks represent, it's impossible to say what they are. Chemists we've talked to suggest they might include other isomers of LSD and material such as lumi-LSD (LSD with an additional oxygen) or potentially unconverted ergotamine.
It is certainly not very reassuring that neither the lab testing the brown microdot nor the British scientist who produced the 1984 UNODC results was able to determine what the second largest peak was in the tested street acid. We are guessing it's an oxidative product or lumi-LSD, but at this point we don't know.
Could those additional peaks be psychoactive by themselves? Could they contribute noticeably to the experience? Most experts don't think so, but there is remarkably little hard data to say with much certainty.
As a side note, if we assume that most of the secondary peaks around the d-LSD peak are lysergic compounds, a rough estimate of the total mass of these would be around 50 µg. It is possible that whoever aliquoted these microdots was working with impure crystal and had intended to make 50 µg doses.
We also received results from an independent lab that had recently tested a brown microdot. Using a cruder testing technique, this lab was able to roughly estimate the dose of the microdots at around 20 micrograms, "give or take 50%". These results were produced using thin layer chromatography and comparing the results against three reference standards of known concentration using a UV light. This technique is not a reliable quantitative method, but it was reassuring that two labs independently arrived at approximately the same value from two different samples of the same dose form.
Because this matches most of the experiential reports we've received and the estimates of experienced LSD users, we believe this quantitative estimate is correct.
Using AUC from the HPLC + UV and rough estimates using TLC, the "brown dot" street samples were estimated to have approximately 21 µg per unit. This value was consistent with widespread reports that effects produced by the brown microdots were "weak".
Erowid has been working for several years to find qualified analytical chemists who have both the time and interest to look at the complex issue of LSD purity and independent quantitative analysis. Over the past three years, we have interacted with only one lab willing and able to perform a detailed quantitative analysis. Out of fear of official scrutiny, they are unlikely to perform any future work. The lab that provided the rough quantitative estimate did so only on the condition that they not be identified.
Independent academic analysis of controlled substances is substantially limited by fears of license revocation or harassment by the DEA. Labs are concerned about cooperating with groups they expect the DEA to disapprove of. The DEA itself has not published scientifically rigorous analyses of street acid and there are unresolved issues about what is sold as acid in the black market.
We are left with the knowledge that, at this time, there is not enough information to resolve the "good acid / bad acid" debate. We will continue to watch for new data on this topic and hope this work can inspire other investigators to look more closely at the issue. Perhaps university researchers will be inspired to contact Erowid about follow-up work.
Although it has now been over 60 years since the discovery of LSD--with thousands of papers published about its chemistry, pharmacology, and social impact--there are still huge questions about what is sold on the street as "acid".
| Glossary |
|
Aliquot – to divide or measure into equal parts.
Isomers – two substances that are composed of the same elements in the same proportions but with a slightly different arrangement of atoms.
d-LSD – (or d-LSD-25) is the known active form of LSD first synthesized by Albert Hofmann.
iso-LSD – an isomer of d-LSD believed to be non-psychoactive.
|
"Good Acid" vs. "Bad Acid"
One of the most persistent ongoing debates about street acid is why people report that different batches are experientially different in their effects. Is it the result of different qualities of acid? Is it the result of different dosages? Or is it simply the result of normal variations in experience from one person to another or one situation to another?Some people believe that there is no pure acid on the street these days: that the last "clean" acid was made by Sandoz or Owsley.2 Others believe that "acid is acid": that differences in purity don't play a significant role in different effects, which are instead explained as the result of differences in dosage, set, setting, and individual reactions. Yet a third opinion is that there are variations in purity--from incomplete syntheses, poorly purified material or degraded material--as well as a variety of chemicals such as ALD-52 or isomers of LSD that might be sold as street acid.
One of the primary arguments against the premise that differences in LSD experiences are the result of differences in quality of material has come from people we've spoken to who have distributed and aliquoted acid in the past. One such person described how some recipients of his LSD would go on at length about how distinct and how much better one type of blotter was than another. Yet, often, both types had been aliquoted by this chemist on the same day, from the same batch of liquid, onto similar blotter paper bearing different designs.
The Legality of Testing
These questions should be trivial to address, yet for several years we have been trying to collect reliable information about the contents of street LSD, with minimal results.Unfortunately, getting street LSD legally analyzed is extremely difficult for anyone outside of law enforcement. Starting in the early 1970s with the passage of the Controlled Substances Act, tight restrictions were put in place over who is allowed to analyze and research controlled substances. Only those labs or individuals who are licensed by the DEA are allowed to handle Schedule I substances such as LSD.
In 1972, the BNDD (The Bureau of Narcotics and Dangerous Drugs) requested that labs collect names and addresses for anyone submitting controlled substances for testing.3 Then in 1974, the DEA issued firm guidelines that required licensed laboratories to stop providing quantitative results for anonymously submitted samples of scheduled substances.4 Now, unless a person is willing to walk in to a lab, give their name, show identification (which would be recorded in the lab's records), and then submit the sample (thereby volunteering proof of having committed a crime), labs are not permitted to provide information about how much of any chemical is present in the sample. Not surprisingly, most lawyers would advise against such an action.
| PharmChem Summary of Street Acid Analysis |
| (1969-1975) |
|
|
| Testing methods were not described. |
Though these rules against testing are only DEA "guidelines" (we do not believe they are part of any law or publicly available official policy), representatives of at least one lab we spoke with have been threatened with having their license revoked after participating in research the DEA didn't like. Since DEA licensing is required in order to handle Schedule I substances, labs that work with such chemicals are understandably reticent to risk their livelihood by going against these guidelines.
The Difficulty of Testing
Testing LSD is technically more difficult than the testing of many other common psychoactives. This is because the dose range is very low and because it's a complex molecule. There are simple tests available for identifying the presence of LSD (using thin layer chromatography or immunoassay). But detecting and characterizing impurities or quantifying the exact amount of LSD in a given dose unit is more complicated and requires an experienced analytical chemist with specialized equipment and an interest in the topic. Unfortunately, because of the combination of DEA restrictions and the difficulty of testing, there may be no labs in the United States currently licensed and qualified to do anonymous testing of LSD.
The Existing Data
The difficulty we encountered in getting currently-circulating samples tested led us to collect what historical information we could find about the contents of street LSD. | Pharmchem Test Results | ||
|---|---|---|
| ( Examples from December 1973 ) | ||
| Alleged Content | Actual Content | Description |
| LSD | 40 µg LSD | white blotter paper |
| LSD | 90 µg LSD + 10 µg iso-LSD | yellow tablet |
| LSD | 60 µg LSD | yellow-green powder |
| LSD | 55 µg LSD + 15 µg iso-LSD | blue tablet, "microdot" |
| LSD | 140 µg LSD + 40 µg iso-LSD | white blotter paper |
| LSD | 65 µg LSD | white double-dome tablet |
| LSD | 55 µg LSD + 10 µg iso-LSD | white blotter paper, "Mr. Natural" |
| LSD | 45 µg LSD | pink spot on white/red/blue blotter |
| LSD | 55 µg LSD | orange tablet |
| LSD | 60 µg LSD | orange tablet |
| LSD | 130 µg LSD | amber gelatin square |
| LSD | 60 µg LSD + 20 µg iso-LSD | magenta spot on white blotter |
| LSD | 100 µg LSD + 10 µg iso-LSD | spot on white blotter paper |
| LSD | 150 µg LSD + 20 µg iso-LSD | spot on white blotter paper |
| LSD | 45 µg LSD | blue tablet |
| LSD | 80 µg LSD + 20 µg iso-LSD | beige spot on white blotter |
| LSD | 60 µg LSD | white tablet |
PharmChem Data
In the early 1970s, prior to issuance of the DEA's guidelines, there were a few labs that actively analyzed street LSD. The most prominent of these labs was PharmChem, based in Palo Alto, California. Between 1972 and 1978, PharmChem solicited users to send in samples of chemicals purchased on the street. They then worked with labs around the country to provide testing results for the submitted samples.Prior to 1974, PharmChem published both qualitative and some quantitative testing results--including microgram dosages for street LSD--in their print newsletter. Each month they would publish a list of hundreds of samples that had been submitted, including information about what the submitter believed the substance to be, where and when it was purchased, how much it had cost, a physical description of the material, and what the test results were for the sample. Unfortunately, they failed to describe their testing methods. We believe, based on talking to Alexander Shulgin and a former PharmChem employee who worked there after their "Analysis Anonymous" program had ended, that they used thin layer chromatography to detect both the presence of LSD, and what could only be estimates of the quantities present.
In March 1977, PharmChem published a review of the street drug analysis they had done in the United States and Canada between 1969 and 1975. Looking at a total of 2200 samples submitted as "acid" or "LSD", they reported that 87% of the submitted samples alleged to be LSD were d-LSD with no other psychoactive drug present. In LSD-positive samples, they found a range of 5 to 500 µg with an average of 75 µg per dose.5
Interestingly, people who submitted samples to PharmChem also often submitted comments about whether they believed that the acid contained "speed" or "strychnine". Although no strychnine was ever detected in any of the submitted samples--and only a few tested positive for methamphetamine--PharmChem reported that the more LSD that was present in a dose unit, the more likely the submitter was to think it contained strychnine.
Following the issuance of the DEA's 1974 guidelines, PharmChem and other labs quickly stopped publishing quantitative data.
| DEA Information about LSD |
| (2003) |
|
|
| Note possible conflicts of interest in reporting. |
DEA Data
The second major source of testing data is the DEA itself. Unfortunately, their law enforcement mission does not necessitate that they provide the public with the vast quantities of information they generate in their pursuit of drug law violators. Though occasionally they publish average dosages for the street acid they confiscate, they are not necessarily neutral in their reporting. Because there is no oversight or external verification of any of their data and because of potential conflicts of interest between science and law enforcement, their data must be considered carefully.According to the DEA, the average dose of LSD on street blotter is between 20 and 80 micrograms (µg). Based on this, they now consider a "standard dose" of LSD to be around 50 µg. They also report that crystal LSD they have seized averages about 62% d-LSD (with the rest made up of other isomers or impurities) but that some is 95-100% pure.6
For more than 35 years, the DEA and their predecessor organizations have produced a private--in fact almost secret--internal publication called Microgram. Prior to 2003, this publication was available only to the DEA and the labs and chemists they worked with. A wide variety of information about the street use of scheduled substances and about law enforcement action has been published in Microgram. Over the years, it has included information about LSD blotter designs tested by DEA offices around the United States and the lab techniques used to test them. They have also regularly printed images of street acid and sometimes included quantitative information.
In 2002, in response to Erowid's interest in learning more about the DEA's past LSD analysis, a Freedom of Information request was filed for a 10-year-old issue of Microgram. The request was denied and is currently being appealed.
Still seeking the information, we were soon informed that some obscure libraries in the United States happen to carry back issues. From these libraries and from Erowid friends, we have been able to piece together a collection of back issues of the publication.
The latest year of Microgram we have access to is 1996. In that year, the DEA published a handful of blotter images and a small number of quantitative test results. The doses they report were between 28 and 78 micrograms per hit of blotter.
More interestingly, in 1987, the DEA published an article in Microgram titled "The LSD Blotter Index" by Franzosa, Harper, and Crockett. This article is a catalog of hundreds of blotter designs seized by law enforcement between 1976 and 1986, including quantitative information for many of them.7 In this index, the reported dose per unit ranges from as low as a couple of micrograms to doses near 200 µg per hit.
Unfortunately, Microgram is not scientifically rigorous in its reporting of the DEA's testing results. No information is provided in The Blotter Index about which labs provided which data and what methods were used for detection or quantification. Likewise, the DEA's data about average doses is difficult to reproduce or verify, as their methods for averaging are not described and they do not provide information about how many doses of each design were produced, seized, or tested.
The dose per unit for individual designs described in The Blotter Index also shows an average of about 20-80 µg per hit. There is speculation that some of this variation might be the result of inconsistency in the dipping process resulting in higher doses on one edge of a sheet than the other. A copy of this article is available in the Erowid LSD Vault.
Other Countries
Other countries have different regulations than the United States regarding testing of controlled substances and some European countries appear to have easier access to quantitative testing. The Netherlands have an ongoing free testing program where individuals can bring in a sample of any street drug to be tested. We were given a summary of their testing results that shows that they receive very few samples of street acid to be tested. Of 600 samples submitted in 2001 and 2002, they tested only 12 samples alleged to be LSD and only half of these actually contained LSD. Of these, they reported doses ranging from 5 to 168 micrograms. Additionally, the person we communicated with from their lab said that results for the two lowest dose samples were considered unreliable. The Dutch lab reports that they use HPLC for their quantification and occasionally use GC/MS to verify results.8
Although we have been unable to get results from or clear documentation about ongoing testing programs in other countries, we believe that testing programs for street drugs exist in France, Spain, Austria, and Switzerland, most funded by the national governments. Most of these programs are oriented towards testing ecstasy tablets, cocaine, or heroin and it is unclear whether they could reliably detect or quantify various forms of LSD, let alone detect minor impurities.
New LSD Testing
After being disappointed by our historical search--finding little to help resolve the enduring debate about what differences exist between batches of street LSD--we continued to seek a lab with the necessary skills to perform LSD analysis. Earlier this year, we were able to find a licensed lab with access to an analytical standard of LSD as well as a lab sample from 10 years earlier that had been stored under nitrogen in freebase form. They also had access to a street sample in the form of a small brown microdot.Information about these brown microdots was reported to Erowid from around the United States between August 2002 and May 2003. Additionally, the newly public version of the DEA's Microgram reported a seizure of two such brown microdots in Owatonna, Minnesota in April 2003.9 Though the Microgram mention did not include quantitative information, most reports (though not all) have described these microdots as "very weak", suggesting a low dosage per tablet.
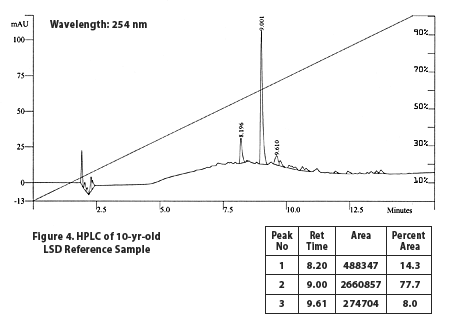
The lab we communicated with used high performance liquid chromatography (HPLC), ultraviolet (UV) absorption measurements, and liquid chromatography/mass spectrometry (LC/MS) to determine what was in the microdot. Using the same methods, the microdot results [Fig. 1] were compared against both the high quality analytical standard [Fig. 2] and the 10-year-old lab sample [Fig. 4].

Testing Methods
The most useful of the tests performed was HPLC followed by UV absorption. For this, the sample is dissolved in a solvent (such as water or methanol) and is then injected into a long, thin tube called a "column". The column has a specially prepared coating on the inside that causes different chemicals to travel at slightly different speeds as they move through the tube. At the end of the column is a detector that senses when the chemicals emerge from the tube. The output from the column is subjected to ultraviolet light at a chosen wavelength. For this test 254 nanometer light was used, a common detection frequency.
The time the sample takes to move through the column is called the "retention time" and is marked along the x-axis (bottom side) of figures 1, 2, and 4.
The y-axis (left side) of the charts shows how much UV light was absorbed by the material coming out of the column at a particular point in time.
Each peak in the chart represents when each chemical component of a sample reached the end of the column. The small bumps nearest the left side of each chart are called "solvent fronts" and are usually ignored because they represent residue chemicals carried through the system with the initial "wave" of the injected solvent.
The HPLC + UV output for the new reference standard of d-LSD (Fig. 1) is quite simple. In the middle of this figure, there is a single very clean, clear peak with a retention time of 9.047 minutes. Using an electro-spray mass spectrometer, the lab verified that this reference standard had the correct molecular weight (323) for d-LSD. This material was further verified by checking that its UV absorption profile had the correct peak absorption at around 320 nanometers.
This all verified that the new reference standard appears to be very pure d-LSD. Although there are some extremely minor perturbations in the baseline around the LSD peak, these are well within the margin of error of the equipment.
What Is in the Microdot?
The HPLC + UV output for the street microdot (Fig. 2) shows a central peak on the graph that is without question d-LSD. Its retention time matches almost exactly that of the reference standard (small variations in retention time are normal) and mass spectrometry verified that d-LSD was present in the sample. Since each peak represents a chemical present in the sample, and there are several significant peaks besides the one known to be d-LSD, that leaves us with the important question--what chemicals do the rest of the peaks represent? Relative amounts of each chemical can be measured by comparing what is known as Area Under the Curve (AUC), shown in the table below the graph.
In figure 2, peak 3 (at 8.12 minutes) is nearly half the area of the d-LSD peak and peak 6 (at 9.5 minutes) is a little over a third of the area of the d-LSD peak. This suggests that the chemicals represented by those peaks are present in the microdot sample in quantities one-half and one-third of the total d-LSD present, respectively. Because the exact retention times for specific substances can vary quite a bit, based on factors such as what type of solvent and column were used, retention time cannot be used by itself to identify a substance.
 We believe that peak 6 is iso-LSD, an isomer of LSD. The lab guessed this peak to be iso-LSD based both on its UV absorption profile (image not shown) and on their analysis of results of their LC/MS work (also not shown).
We believe that peak 6 is iso-LSD, an isomer of LSD. The lab guessed this peak to be iso-LSD based both on its UV absorption profile (image not shown) and on their analysis of results of their LC/MS work (also not shown).This is also confirmed by a 1984 article published by the United Nations Office on Drugs and Crime,10 which discussed analytical work done in the United Kingdom. Figure 3 is an image from this paper showing the HPLC + UV profile for a street sample tested in 1984. The similarities to the results from the brown microdot are striking. This paper identifies peak 4 (the equivalent to peak 5 in the microdot sample) as d-LSD, peak 5 (equivalent to microdot peak 6) as iso-LSD, peak 3 (equivalent to microdot peak 4) as most likely unconverted ergotamine, and says peaks 1 and 2 are unidentified.
Iso-LSD is considered non-psychoactive. Papers in the late 1950s reported low or no activity for iso-LSD, including a very interesting report published by Sandoz comparing activities of several "lysergic acid derivatives".11 Albert Hofmann reported having tried iso-LSD at doses up to 500 µg and found no psychoactivity. More recent rat experiments have found that rats given iso-LSD in a discrimination study don't respond as though it's d-LSD.12 It is an open question, however, whether high levels of iso-LSD could possibly alter the effects of LSD, either through activity at a different serotonin receptor type than d-LSD triggers or through some other mechanism.
As far as we know, no research has been done to determine whether isomers of LSD can potentially reduce or alter the receptor binding or otherwise modify the pharmacodynamics of d-LSD. At this point, iso-LSD is mostly believed to be inert and simply represents "wasted" lysergic material since it is in the wrong configuration to be psychoactive.

10-Year-Old Lab Sample
The last HPLC + UV graph (Fig. 4) shows a lab sample of LSD freebase that had been stored under nitrogen for more than ten years. The lab said that the sample had started as extremely pure d-LSD but did not have any record of previous analysis results. We can see that the number of secondary peaks is far lower than with the brown microdot. The three tallest peaks appear to coincide with the three tallest peaks in the brown dot, and the 9.0 peak was confirmed to have the correct UV absorption pattern for d-LSD. The lab believed the peak at 9.6 to be iso-LSD based on the UV and mass spectrums.The rest of the small peaks in that area of the graph are likely other lysergic-structure chemicals, although without doing work to characterize exactly what those peaks represent, it's impossible to say what they are. Chemists we've talked to suggest they might include other isomers of LSD and material such as lumi-LSD (LSD with an additional oxygen) or potentially unconverted ergotamine.
It is certainly not very reassuring that neither the lab testing the brown microdot nor the British scientist who produced the 1984 UNODC results was able to determine what the second largest peak was in the tested street acid. We are guessing it's an oxidative product or lumi-LSD, but at this point we don't know.
Could those additional peaks be psychoactive by themselves? Could they contribute noticeably to the experience? Most experts don't think so, but there is remarkably little hard data to say with much certainty.
Quantity
Finally, by having a reference standard of known concentration, it is possible to interpolate the dose of d-LSD on the single brown microdot that was tested. By using the Area Under the Curve for the reference standard and the total amount of d-LSD injected for that result, in this case 1.49 µg, then comparing the total areas under the d-LSD peaks (in this case, the brown dot's result is about .28 the total area of the reference standard's peak), and finally dividing this by the portion of the street sample that was injected (in this case 2%), we can estimate the total quantity of d-LSD in the brown dot. According to this analysis, with a margin of error of about 10%, the brown microdot contains 21 µg of d-LSD per tablet. As a side note, if we assume that most of the secondary peaks around the d-LSD peak are lysergic compounds, a rough estimate of the total mass of these would be around 50 µg. It is possible that whoever aliquoted these microdots was working with impure crystal and had intended to make 50 µg doses.
Confirmation of Results
After hearing that we were interested in learning more about the contents of street LSD, an anonymous individual contacted us and volunteered to send a brown microdot to a lab for testing. This microdot was tested by a DEA-licensed lab that positively identified d-LSD in the sample. We also received results from an independent lab that had recently tested a brown microdot. Using a cruder testing technique, this lab was able to roughly estimate the dose of the microdots at around 20 micrograms, "give or take 50%". These results were produced using thin layer chromatography and comparing the results against three reference standards of known concentration using a UV light. This technique is not a reliable quantitative method, but it was reassuring that two labs independently arrived at approximately the same value from two different samples of the same dose form.
Because this matches most of the experiential reports we've received and the estimates of experienced LSD users, we believe this quantitative estimate is correct.
Summary
The results of these analyses showed that the "brown dot" samples of street acid contained d-LSD, as well as several other major components. One of these substances was identified as iso-LSD, based on molecular weight, UV absorption pattern, and similarity to published HPLC results of a street LSD sample. Another component was suggested to be lumi-LSD, but a clear molecular weight was not obtained and no reference standard for lumi-LSD was available. Three other chemicals present in the sample, making up about 15 percent of the total detected material, have not been identified. Using AUC from the HPLC + UV and rough estimates using TLC, the "brown dot" street samples were estimated to have approximately 21 µg per unit. This value was consistent with widespread reports that effects produced by the brown microdots were "weak".
Erowid has been working for several years to find qualified analytical chemists who have both the time and interest to look at the complex issue of LSD purity and independent quantitative analysis. Over the past three years, we have interacted with only one lab willing and able to perform a detailed quantitative analysis. Out of fear of official scrutiny, they are unlikely to perform any future work. The lab that provided the rough quantitative estimate did so only on the condition that they not be identified.
Independent academic analysis of controlled substances is substantially limited by fears of license revocation or harassment by the DEA. Labs are concerned about cooperating with groups they expect the DEA to disapprove of. The DEA itself has not published scientifically rigorous analyses of street acid and there are unresolved issues about what is sold as acid in the black market.
We are left with the knowledge that, at this time, there is not enough information to resolve the "good acid / bad acid" debate. We will continue to watch for new data on this topic and hope this work can inspire other investigators to look more closely at the issue. Perhaps university researchers will be inspired to contact Erowid about follow-up work.
References #
- National Survey on Drug Use and Health (formerly the National Household Survey on Drug Abuse). Substance Abuse and Mental Health Services Administration, 2002 data. Retrieved from samhsa.gov/oas/nhsda.htm
- Eisner B. "LSD Purity: Cleanliness is Next to Godheadliness." High Times. Jan 1977; 17:73-75.
- PharmChem Newsletter. 1972; 1(2):1.[excerpt]
- PharmChem Newsletter. 1974; 3(4):1.
- "Street Drug Analysis and Drug Use Trends 1969-1975." PharmChem Newsletter. 1977; 6(3):2.
- Drug Enforcement Administration. "LSD Manufacture." DEA Website Intelligence Reports. Retrieved May 2003 from www.usdoj.gov/dea/pubs/lsd/lsd-5.htm
- Franzosa, Harper, and Crockett. "The Blotter Index." Microgram. Jul 1987; 10(7).
- Vervaeke H. Personal Communication. Mar 2003. drugsinfo.nl/testen/
- Drug Enforcement Administration. Microgram. Apr 2003; 36(4).
- Humphreys IJ. "The Work of the Drugs Intelligence Laboratory, Home Office Forensic Science Service." Jan 1984. Retrieved from unodc.org/unodc/en/bulletin/bulletin_1984-01-01_1_page005.html
- Pharmacologic Properties and Psychotogenic Effects of some Lysergic Acid Derivatives: Comparison with Delysid (LSD-25). Internal Sandoz Publication, 1958. Retrieved from erowid.org/references/refs_view.php?id=6084
- Anonymous. Personal Communication. 2003.


