by Erowid
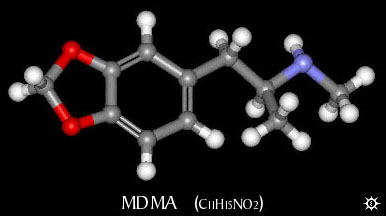
| NAME : | MDMA |
| CHEMICAL NAME : | 3,4-methylenedioxymethamphetamine |
| ALTERNATE CHEMICAL NAMES : |
3,4-methylenedioxy-n-methylamphetamine; N, alpha-Dimethyl-1,3-benzodioxole-5-ethanamine; methylenedioxy-n-methylamphetamine; N-methyl-3,4-methylenedioxyphenylisopropylamine; Ecstasy; E; XTC; Adam |
| CHEMICAL FORMULA | C11H15NO2 |
| MOLECULAR WEIGHT | 193.25 |
| MELTING POINT | 147-148° C (Hydrochloride crystals from isopropanol/n-hexane) |
| MELTING POINT | 152-153° C (Hydrochloride crystals from isopropanol/ether) |
| LD50 | (mice) 97 mg/kg i.p. |
| LD50 | (rats) 49 mg/kg i.p. |
| LD50 | (guinea pigs) 98 mg/kg i.p. |
| From the Merck Index 12th Edition | |
|---|---|
INFORMATION
MDMA Material Safety Data Sheet (Radian)
Pihkal MDMA Synthesis
Compare MDMA molecule to another chemical
OFF-SITE LINKS
PubChem : MDMA
MERCK INDEX ENTRY
5806. MDMA. N, alpha-Dimethyl-1,3-benzodioxole-5-ethanamine; 3,4-methylenedioxymethamphetamine; N-methyl-3,4-methylenedioxyphenylisopropylamine; "Ecstasy"; "E"; "XTC"; "Adam". C11H15NO2; mol wt 193.25. C 68.37%, H 7.82%, N 7.25%, O 16.56%. Entactogen; N-methyl derivative of MDA, q.v. (S)-Form possesses more potent CNS activity. Prepn: Ger. pat. 274,350 (1914 to E. Merck), C.A. 8, 3350 (1914); U. Braun et al., J. Pharm.Sci. 69, 192 (1980). Spectral and chromatographic analyses: K. Bailey et al., J. Assoc. Offic. Anal. Chem. 58, 62 (1975). GC/MS determn in urine: B.K. Gan et al., J. Forensic Sci. 36, 1331 (1991). HPLC determn in whole blood: R. E. Michel et al., J. Neurosci. Methods 50, 61 (1993). Acute toxicity study: H. F. Hardman et al., Toxicol. Appl. Pharmacol. 25, 199 (1973). Series of articles on history, pharmacology, and neurotoxicity: : Ann. N.Y. Acad. Sci. 600, 601-715 (1990). Review of biochemical actions: M. Rattray, Essays Biochem. 26, 77-87 (1991); of pharmacology, toxicology, and human experience: T.D. Steele et al., Addictions 89, 539-551 (1994); A.R. Green et al., Psychopharmacology 119, 247-260 (1995).
Hydrocholoride, C11H15NO2.HCl, crystals from isopopanol/n-hexane, mp 147-148° (Bailey). Crystals from isopropanol/ether, mp 152-153° (Braun). uv max (ethanol): 286 nm (E 2843). LD50 in mice, rats, guinea pigs (mg/gk): 97,49,98 i.p. (Hardman).
Note: This is a controlled substance (hallucinogen) listed in the U.S. Code of Federal Regulations, Title 21 Part 1308.11 (1995).


