Neurology, Dec. 1998
Karen I. Bolla, PhD; Una D. McCann, MD; and George A. Ricaurte, MD, PhD
OCR & HTML by Erowid
INDEX
ARTICLE ABSTRACT
BackgroundMethylenedioxymethamphetamine (MDMA, or "Ecstasy") is a popular recreational drug of abuse that is known to damage brain serotonergic neurons in animals and possibly humans. Few functional consequences of MDMA-induced serotonin (5-HT) neurotoxicity have been identified, either in animals or humans. This study sought to determine whether individuals with a history of extensive MDMA use showed evidence of memory impairment, because brain serotonin has been implicated in mnemonic function.
Method: The authors compared 24 abstinent MDMA users and 24 control subjects on several standardized tests of memory, after matching subjects for age, gender, educational level, and vocabulary score (a surrogate of verbal intelligence). The authors also explored correlations between changes in memory function and decrements in CSF 5-hydroxyindoleacetic acid (5-HIAA), which serves as a marker of central 5-HT neural function.
Results: Greater use of MDMA (total milligrams per month) was associated with greater impairment in immediate verbal memory (p ‹ 0.02) and delayed visual memory (p ‹ 0.06). Furthermore, lower vocabulary scores were associated with stronger dose-related effects, with men having greater dose-related deficits than women. Lastly, lower concentrations of CSF 5-141AA were associated with poorer memory performance. Conclusion: Abstinent MDMA users have impairment in verbal and visual memory. The extent of memory impairment correlates with the degree of MDMA exposure and the reduction in brain 5-HT, as indexed by CSF 5-111AA.
Recreational use of the ring-substituted amphetamine derivative ± 3,4-methylene-dioxymethamphetamine (MDMA, or "Ecstasy") has increased significantly in recent years,[1,2] particularly in the setting of large, organized social gatherings known as raves.[3] The most recent survey in the United States estimated that 2.3% of college students and 4.3% of young adults (19 to 28 years) used MDMA at least once during the preceding year.[2] Several experimental studies indicate that MDMA damages brain serotonin (5-HT) neurons in animals (including nonhuman primates) and possibly humans.[4-11] These findings raise concern that the growing number of recreational MDMA users may be at risk for incurring brain serotonin neurotoxicity. This concern is accentuated by the fact that some MDMA users take doses that are equivalent to those demonstrated to be neurotoxic in animals,[9] particularly after established principles of interspecies dose scaling are considered.[12]
Few functional consequences of MDMA-induced brain 5-HT neurotoxicity have been identified, either in animals or humans.[13] At present, it is not clear whether the apparent paucity of functional consequences of MDMA-induced 5-HT neurotoxicity is secondary to true absence of measurable consequences, or whether it is because of the lack of studies addressing this issue. The lack of sufficiently specific and valid measures of brain 5-HT function may also play a role.
Memory function deserves special scrutiny in MDMA-exposed individuals for several reasons. First, 5-HT appears to play a role in mnemonic function.[14-16] Second, in animals, MDMA severely damages 5-HT axons in the hippocampus and other brain regions implicated in learning and memory (e.g., thalamus ).[7,10,17,18] Third, case reports of memory impairment in some MDMA users,[19] and several studies, [19-21] suggest that MDMA users have impaired verbal memory function. Because previous studies have involved subjects who may have recently used MDMA or other centrally acting drugs (see Discussion), it is not entirely clear whether deficits in MDMA users represent neurotoxic effects of MDMA, pharmacologic effects of drugs, or drug withdrawal.
The purpose of the current study was to determine whether memory deficits exist in MDMA users who were drug free for at least 2 weeks, and if they do, whether memory deficits are dose related. Also, this study examined whether memory deficits in MDMA users correlate with decrements in CSF 5-hydroxyindoleacetic acid (5-HIAA), [22,23] which serves as a reliable indicator of MDMA-induced brain 5-HT neurotoxicity in nonhuman primates.[24] This study was part of a larger clinical research project assessing the long-term effects of MDMA in humans.
METHODS
assuming 100 mg is equal to one capsule. † Usual dose (mg) multiplied by frequency per month. | ||||||||||||||||||||
Thirty individuals with a history of MDMA use and 28 control subjects participated in the overall protocol examining the neurotoxic potential of MDMA in humans and its functional consequences. Forty-eight of these subjects (24 control subjects and 24 MDMA users) were included in the current study to facilitate matching of the two study groups. Individuals were excluded if they were older than 50 years or their raw vocabulary score was less than 24 or greater than 67. Subjects were also excluded if English was not their native language, because the neurobehavioral tests used herein have not been validated for non-English-speaking individuals. Subjects that used MDMA were self-referred, having learned about ongoing research on the consequences of MDMA use. All MDMA subjects had used MDMA on at least 25 separate occasions by self-report. Control subjects were recruited by local advertisements and had no self-reported prior use of MDMA. Because most MDMA users also experimented with other recreational drugs, prior use of recreational drugs other than MDMA was also allowed for control subjects. Had drug-naive control subjects been used instead, it would have been difficult to attribute group differences to MDMA use rather than to drug use in general. Exclusions for both groups included past or current history of major medical illness (e.g., neurologic, renal, endocrine, or hematologic), current major psychiatric illness as determined by Structured Clinical Interview for DSM-III-R (SCID-111-R), a positive drug screen for illicit or prescribed psychoactive drugs, or current alcohol abuse or dependence. Subjects agreed to abstain from all recreational drugs for at least 2 weeks before testing. Their drug-free status was confirmed by urine and blood drug screens. Given that the half-life of MDMA in animals is 1 to 2 hours[25] the 2-week period of abstinence was deemed long enough to rule out any withdrawal effects. Participants were paid for their time and travel expenses. Informed consent was obtained from all participants and the research protocol was approved by the institutional review board.
Procedure
Participants were admitted for a 5-day inpatient stay at the Johns Hopkins Bayview Clinical Research Center, where they underwent physical examina-ions, blood and urine laboratory testing, EKGs, and structured diagnostic psychiatric interviews using the SCID-III-R.
Memory Testing
Tests of memory included the Wechsler Memory Scale-Revised (WMS-R),[26] the Rey Auditory Verbal Learning Test (RAVLT)[27] and the Rey-Osterrieth Complex Figure (RCF).[28,29] The WMS-R is composed of 13 subtests that measure different aspects of memory. The RAVLT is a verbal memory test and the RCF is a visual memory test. The Wechsler Adult Intelligence Scale -Revised (WAIS-R) vocabulary subtest was also administered as an estimate of verbal intelligence.[30] In previous studies of lead, solvents, and aging, the WAIS-R vocabulary subtest score was relatively insensitive to the effects of neurotoxins and aging[31] and was a better predictor of neurobehavioral performance than level of education.[32]
CSF 5-HIAA concentrations
Concentrations of CSF 5-HIAA were determined by high-performance liquid chromatography coupled with electrochemical detection, as described previously.[18]
group who reported any prior use of a drug in the listed drug class. MDMA = methylenedioxymethamphetamine; LSD = lysergic acid diethylamide; PCP = phencyclidine. | ||||||||||||||||||||||||||||||||||||||||||||
Detailed information about prior MDMA use was obtained from a structured interview that ascertained the number of milligrams per capsule of MDMA generally taken at one time (each capsule was assumed to equal 100 mg), the number of times that MDMA was taken per month, and the total number of months of MDMA use. These variables provided an estimate of the intensity, frequency, and duration of MDMA use. A dose variable--a combination of intensity and frequency -- was calculated by multiplying the self-reported milligrams ingested in a single MDMA session (which could last hours and involve several separate doses of MDMA) by the number of MDMA sessions per month. Table 1 summarizes the characteristics of MDMA use. Table 2 shows drug histories of both subject groups, ascertained from a standard drug history questionnaire.
Statistical analysis
To assess possible effects of MDMA exposure on specific memory functions, the 15 individual memory tests were reduced to four memory factors (immediate verbal memory, immediate visual memory, delayed verbal memory, and delayed visual memory), based on the specific memory function measured by the test.[33] The immediate verbal memory factor was made up of RAVLT logical memory, digit span total, and verbal paired associates. The delayed verbal memory factor incorporated RAVLT-recall, RAVLT-recognition, logical memory-recall, and verbal paired associates-recall. The immediate visual memory factor was made up of the RCF, visual reproduction, visual paired associates, and figural memory, whereas the delayed visual memory factor incorporated RCF-recall, visual paired associates-recall, and visual reproduction-recall. Due to differences in maximal possible scores on each subtest, individual test scores were converted into z scores using the means and SDs of the control group, summed and divided by the number of tests in each memory factor. Separate multiple linear regressions for each of the four memory factors were used to explore the relation between MDMA use and memory performance. Subject groups were matched for age, gender, education level, and WAIS-R vocabulary subtest scores. Although groups were matched on demographic variables, previous studies indicate that age, vocabulary score, and gender can influence performance on neurobehavioral tests. Therefore, initial exploratory analyses included these independent variables in the models. Independent variables were retained in the model if associated significantly with multiple outcomes (p ‹ 0.05). Interaction terms (i.e., age × group or dose; vocabulary × group or dose; and gender × group or dose) were also examined. Also, because neurotoxic agents such as lead and solvents affect the nervous system predominately at higher doses [31,34] a second set of analyses was performed by substituting estimated MDMA dose (milligrams per month) for a group in the models. All statistical analyses were performed with the BMDP statistical software program (version 7.0; Biomedical Statistical Software, Los Angeles, CA).[35]
RESULTS
| |||||||||||||||||||||||||||||||||||||||
The control and MDMA groups were similar in age, educational level, and WAIS-R vocabulary subtest score (table 3).
Regression analyses
Separate multiple linear regressions examined the associations between MDMA use and memory performance for each of the four memory factors examined. Age was not related to any memory factor and was therefore dropped from the regression models. Conversely, vocabulary score and gender, which were highly predictive of performance for some of the memory factors, were retained in the regression models.
As might be anticipated, when memory function in the two groups was compared without taking the average monthly MDMA dose into account, differences were not found. However, when the estimated monthly dose (milligrams per dose × frequency of use per month) was included in the regression models (together with vocabulary score and gender), dose was associated with impaired immediate verbal memory (p ‹ 0.04) and delayed visual memory (p ‹ 0.02; table 4). The association of dose and delayed verbal memory approached significance (p ‹ 0.06). Overall, the R² value (the amount of variance in the outcome variable explained by the model) ranged from 0.29 to 0.54. No significant associations were found between the memory factors and other estimates of exposure, including duration of MDMA use or the cumulative lifetime use of MDMA.
Interaction effects
There were two significant interactions involving dose. A dose × vocabulary score interaction was found for delayed visual memory (p ‹ 0.03), and the interaction approached significance for delayed verbal memory (p ‹ 0.09; see table 4). Individuals with lower vocabulary scores had greater decrements in performance with increasing MDMA dose, whereas individuals with higher vocabulary scores had fewer decrements with increasing MDMA dose. Dose × gender interactions existed for delayed verbal memory (p ‹ 0.04) and delayed visual memory (p ‹ 0.04), with women showing fewer decrements in memory performance with increasing dose relative to men. To visualize the dose × vocabulary interaction, MDMA doses were divided into terciles, and the mean for each tercile was used for the dose × vocabulary adjusted plot (figure 1). Low and high vocabulary groups were formed by dividing the group by the median vocabulary score (59.5).
Because associations were found between MDMA dose and decrements in memory performance for the four memory factors, separate multiple linear regressions were performed on each of the individual tests comprising each factor. Again, vocabulary score, gender, dose × vocabulary score, and dose × gender interaction terms were included in the models. As found previously, vocabulary score and gender were highly associated with the majority of the memory tests. After controlling for the confounding effects of vocabulary score and gender, significant associations were found between dose and performance on immediate verbal paired associates (p ‹ 0.03), delayed verbal paired associates (p ‹ 0.01), and delayed visual paired associates (p ‹ 0.01). For all these tests, as dose increased, performance decreased. A dose × vocabulary score interaction was found for immediate visual paired associates (p ծ 0.01). Again, the dose-related effect was more pronounced in the low vocabulary group. Dose × gender interactions were found for immediate verbal paired associates (p ‹ 0.01), immediate visual paired associates (p ‹ 0.03), delayed visual paired associates (p‹ 0.02), and delayed RAVLT recognition (p ‹ 0.02), with women less affected by increasing dose than men.
Relation to CSF 5-HIAA
A separate analysis was conducted to determine the relation among CSF 5-HIAA, average total MDMA dose per month, and memory function. Concentrations of CSF 5-HIAA were corrected for age and height before the analysis. As reported previously,[18] the mean concentration of CSF 5-HIAA was lower in MDMA users compared with control subjects (mean ± SD, 10.3 ± 3.22 ng/mL versus 14.3 ± 7.0 ng/mL; t[45] = 2.47; p ‹ 0.02). Additionally, a negative association was found between MDMA dose (mg/mo) and CSF 5-HIAA concentration (R=-0.519, p ‹ 0.013). As the dose increased, 5-HIAA concentration decreased. CSF 5-HIAA levels were not related to vocabulary scores.
Lastly, the associations between CSF 5-HIAA concentrations and memory performance were determined. After controlling for vocabulary score and gender, the CSF 5-HIAA concentration was associated significantly with the delayed visual memory factor (B=0.04, standard=0.02, p ‹ 0.03, R²=0.23). The lower the concentration of 5-HIAA, the lower the performance. For the individual memory tests, associations between 5-HIAA level and performance were found for immediate figural memory (p ‹ 0.03) and delayed visual reproduction (p ‹ 0.02). Again, the lower the CSF 5-HIAA level, the lower the performance.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DISCUSSION
The main finding of the current study is that abstinent MDMA users have a deficit in visual and verbal memory, and that higher average monthly doses of MDMA are associated with greater decrements in memory function. Furthermore, our results indicate that lower levels of CSF 5-HIAA -- an indirect measure of central 5-HT function -- are associated with poorer memory performance, suggesting that MDMA-induced brain 5-HT neurotoxicity may account for memory impairment in MDMA users. Lastly, our results indicate that both baseline intelligence and gender influence the effects of MDMA on memory function.
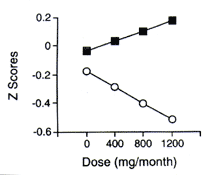 |
|
Figure 1. Relation between methylenedioxy- methamphetamine dose and visual memory delayed for the high vocabulary (square) and low vocabulary (circle) groups (adjusted for gender). A z score of zero represents the mean performance score of the control group, whereas a z score of -0.5 is a value that is 0.5 SDs below the mean performance score of the control group. Therefore, the lower the score, the poorer the performance. The figure reveals a dose × vocabulary score interaction. |
These findings are generally consistent with reports of memory problems in previous studies,[19-21] although some important differences are evident. In particular, contrary to findings in a previous report[20] in which individuals with low MDMA exposure (10 or fewer doses) demonstrated memory deficits, only subjects with high total monthly MDMA dosages were found to have memory deficits in the current study. Discrepancies between the two studies may be attributed in part to the fact that subjects in our study abstained from psychoactive (including MDMA) drugs for at least 2 weeks. Thus, acute or partial residual drug effects, or drug withdrawal, may have caused the memory disturbances noted in previous studies.[19-21] Alternatively, subjects in the study by Parrott et al.[20] may have used extremely high doses of MDMA, causing brain 5-HT neurotoxicity despite the small number of separate drug exposures. Because some individuals attending raves report using doses of MDMA that are clearly neurotoxic in nonhuman primates[23] the latter possibility cannot be excluded.
As reported previously,[18] CSF 5-HIAA concentrations were lower in MDMA users compared with control subjects. Reduced concentrations of CSF 5-HIAA in human MDMA users are likely to reflect MDMA-induced brain 5-HT neurotoxicity because similar reductions have been documented in primates with documented MDMA-induced 5-HT injury.[24] Furthermore, the negative correlation between average monthly dose of MDMA used and CSF 5-HIAA concentrations suggests that MDMA users' self-reports of MDMA use are accurate, because dose-related reductions in brain 5-HIAA have been documented in animals.[4-10] Lastly, because reductions in CSF 5-HIAA levels were associated with lower performance on specific memory tests, our findings support the notion that cognitive deficits in MDMA users may at least be attributed partially to MDMA-induced 5-HT deficits.
The observation that higher exposures to MDMA are associated with memory impairment is consistent with findings in animals, indicating that higher dosages of MDMA produce greater neurotoxic lesions.[10] Notably, only individuals with more profound decrements in CSF 5-HIAA (presumably reflecting a greater extent of 5-HT injury) demonstrated detectable difficulties with memory function. These results are in keeping with a growing body of literature that indicates that large (>80%) lesions of neural systems are often necessary for functional deficits to be evident.[36]
Results from our study also indicate that individuals with lower intellectual abilities (i.e., vocabulary scores) show greater decrements in memory perfo-mance with higher doses of MDMA. Similar interactions are observed in individuals exposed to other neurotoxins such as solvents[34] and aluminum.[37] This effect may be explained by the concept of cognitive reserve, which posits that individuals with higher intellect have a higher threshold for developing neurocognitive effects after brain insult.[38]
Women were less susceptible than men to the MDMA dose-related decrease in memory function. This gender difference may be related to differences in innate cognitive abilities, bioavailability, hormone profiles, pharmacodynamic responses, or differences in baseline memory function. Studies in adults 19 to 50 years old have found that women tend to have better memory abilities, whereas men tend to have better reasoning abilities.[39] Our results are consistent with these reports because gender differences were also seen in control subjects, with women per-forming better than men. Differences between men and women in neurocognitive abilitiesmay be related to differences in cerebral metabolism[40] hemispheric specialization[41,42] or hormonal influences.[43]
Several potential limitations of the current study should be mentioned. First, as with all retrospective studies, there is a possibility that pre-existing differences between MDMA users and nonusers underlie differences in memory function and 5-HIAA. Thus, people with low CSF 5-HIAA may be predisposed to use MDMA and to have memory problems. The dose-related decreases in both CSF 5-HIAA (similar to those that have been found in nonhuman primates) and memory function make this unlikely. Furthermore, because several subjects in the control group also used recreational drugs (albeit not MDMA), a propensity to use drugs cannot fully explain the biological and behavioral differences found in MDMA users in our study. Nevertheless, no single line of evidence can be taken as conclusive proof that MDMA is neurotoxic in humans. Additional studies and converging lines of evidence are needed to better delineate the neurotoxic potential of MDMA in humans and its functional consequences.
ACKNOWLEDGEMENTS
The authors thank Drs. Jean Lud Cadet and Katherine Bonson, National Institute on Drug Abuse-Intramural Research Program, for their critical review and comments.
REFERENCES
- Johnston L, Bachman J, O'Malley P. National survey results on drug use from the Monitoring the Future Study. National Institutes on Drug Abuse, 1975-1994. Vol. 2. College students and young adults. National Institutes of Health (NIH) publication no. 96-4027. Rockville, MD: NIDA, 1996.
- Johnston L, Bachman J, O'Malley P. National survey results on drug use from the Monitoring the Future Study. National Institutes on Drug Abuse, 1975-1995. Vol. 2. College students and young adults. National Institutes of Health (NIH) publication no, 98-4140. Rockville, MD: NIDA. 1997.
- Saunders N. Ecstasy and the dance culture. London: Nicholas Saunders, 1995.
- Stone DM. Stahl DS, Hanson GL, Gibb JW. The effects of 3,4-methyIenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine on monoaminergic systems in the rat brain. Eur J Pharmacol 1986;128:41-48.
- Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymeth-amphetamine. J Pharm Exp Ther 1987; 240:1-7.
- Commins DL, Axt KJ, Vosmer G, Sciden LS. Endogenously produced 5.6-hydroxytryptamine may mediate the neurotoxic effects of parachloroamphetamine. Brain Res 1987;419:253-261.
- O'Hearn G, Battaglia G, DeSouza EB, Kuhar MJ, Molliver ME. Methylenedioxymethamphetamine (MDMA) and methylenedioxymethamphetamine (MDMA) cause ablation of serotonergic terminals in forebrain: immunocytochemical evidence. J Neurosci 1988;8:2788-2803.
- Ricaurte GA, DeLanney LE, Irwin I, Langston JW. Toxic effects of 3,4-methylenedioxymeth-amphetamine (MDMA) on central serotonergic neurons in the primate: importance of route and frequency of drug administration. Brain Res 1988;446:165-168.
- Ricaurte GA, Forno LS, Wilson MA, et al. MDMA selectively damages central serotonergic neurons in nonhuman primates. JAMA 1988;260:51-55.
- Steele T, McCann UD, Ricaurte GA. 3,4-Methylenedioxymethamphetamine (MDMA, "Ecstasy"): pharmacology and toxicology in animals and humans. Addiction 1994;89:539-551.
- McCann UD, Ricaurte GA. Lasting neuropsychiatric sequelae of (±) methlylenedioxymeth-amphetamine (Ecstasy) in recreational users. J Clin Psychopharmacol 1991;11:302-305.
- Mordenti J, Chappell W. The use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V, eds. Toxicokinetics in new drug development. New York: Pergamon Press, 1989:42-96.
- Ricaurte GA, Sabol KE, Seiden LS. Functional consequences of toxic amphetamine analog exposure. In: Cho A, Segal D. eds. Amphetamine and its analogs: neuropsychopharmacology, toxicology. and abuse. New York: Academic Press, 1994: 297-309.
- McEntee WJ, Crook TH. Serotonin, memory, and the aging brain. Psychopharmacology 1991;103:143-149.
- Altman HJ, Normile HJ. What is the nature of the role of the serotonergic nervous system in learning and memory: prospects for development of an effective treatment strategy, for senile dementia. Neurobiol Aging 1988;9:627-638.
- Hunter AJ. Serotonergic involvement in learning and memory. Biochem Soc Trans 1988;17:79-81.
- Squire LR. Neural organization and behavior. In: Plum F, ed, Handbook of physiology. Vol. 5. Washington, DC: American Physiological Society, 1987:295-371.
- McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after (--)3,4-methylenedioxymethamphetamine (MDMA; "Ecstasy"): a controlled study in humans. Neuropsy-chopharmacology 1994:1012):129-138-
- Krystal JH, Price LH, Opsahl C, Ricaurte GA, Heninger GR. Chronic 3,4-methylenedioxy-methamphetamine (MDMA) use: effects on mood and neuropsychological function. Am J Drug Alcohol Abuse 1992;18;331-341.
- Parrot AC, Lees A, Garnham NJ, Jons M, Wesnes K. Cognitive performance in recreational users of MDMA or Ecstasy: evidence for memory deficits. J Psychopharmacol 1998; in press.
- Curran HV, Travill RA. Mood and cognitive effects of ۭ,4-methylenedioxymethamphetamine (MDMA, "Ecstasy"): weekend "high" followed by mid-week low. Addiction 1997;92:821-831.
- Linnoila M, Virkkunen M, Scheinin M, Nautila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations differentiates impulsive from nonimpulsive violent behavior. Life Sci 1984;44:2609-2614.
- Roy A, Adinoff B, Linnoila M. Acting out hostility in normal volunteers: negative correlation with levels of 5-HIAA in cerebrospinal fluid. Psychiatry Res 1988;24:187-194.
- Ricaurte GA, DeLanney LE, Wiener SG, Irwin I, Langston JW. 5-Hydroxyindoleacetic acid in cerebrospinal fluid reflects serotonergic damage induced by 3,4-methylenedioxymethamphetamine in CNS of nonhuman primates. Brain Res 1988;474:359-363.
- Cho KC, Kumagai Y. Metabolism of amphetamine and other arylisopropylamines. In: Cho AK, Segal DS, eds. Amphetamine and its analogs: psychopharmacology, toxicology, and abuse. New York: Academic Press, 1994:43-77.
- Wechsler D. Wechsler Memory Scale-Revised (WMS-R). New York: The Psychological Corporation, 1987).
- Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France, 1964.
- Rey A. L'examen psychologie dans les cas d'encephalopathie traumatique. Arch Psychol 1941;28:286-340.
- Osterrieth PA. Le test de copie d'une figure complex: contribution a l'etude de la perception et de la memoire. Arch Psychol 1944; 30:286-356.
- Wechsler D. Wechsler Adult Intelligence Scale-REvised (WAIS-R). New York: The Psychological Corporation, 1981.
- Bolla KI, Schwartz BS, Stewart W, Rignani J, Agnew J, Ford DP. A comparison of neurobehavioral function in workers exposed to solvents. Am J Industr Med 1995;27(2):231-246.
- Bolla-Wilson K, Bleecker ML. The influence of verbal intelligence, sex, age and education on the Rey Auditory Verbal Learning Test. Dev Neuropsychol 1986;2(3):203-211.
- Lezak MD. Neuropsychological assessment. New York: Oxford University Press, 1995.
- Bleecker ML, Bolla KI, Agnew J, Ford DP, Schwartz BS, Powell S. Dose-related subclinical neurobehavioral effects of chronic exposure to low levels of organic solvents. Am J Industr Med 1991;19:715-728.
- Dixon WJ, ed. BMDP statistical software manual. Berkeley, CA: University of California Press, 1992.
- Sabel BA. Unrecognized potential of surviving neurons: within systems plasticity, recovery of function, and the hypothesis of minimal residual structure, Neuroscientist 1997;3:366-370.
- Bolla KI, Briefel G, Spector D, et al. Neurocognitive effects of aluminum. Arch Neurol 1992;49:1021-1026.
- Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 1993;7:272-295.
- Stumpf H, Jackson DN. Gender-related differences in cognitive abilities: evidence from medical school admissions testing program. Person Individ Differences 1994;17:335-344.
- Esposito G, Van Horn JD, Wienberger DR, Berman KF. Gender differences in cerebral blood flow a a function of cognitive state with PET. J Nucl Med 1996;37:559-564.
- Azari NP, Pettigrew ND, Pietrini P, Murphy DG, Horwitz B, Schapiro MB. Gender differences in patterns of hemispheric cerebral metabolism: a multiple regression/discriminant analysis of positron emission tomographic data. Int J. Neurosci 1995;81:1-20.
- Matthew RJ, Wilson WH, Tant SR. Determinants of resting regional cerebral blood flow in normal subjects. Biol Psychiatry 1986;21:907-914.
- Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and risk of developing Alzheimer's disease: The Baltimore Longitudinal Study of Aging. Neurology 1997;48:1517-1521.
- Saunders N. Ecstasy and the dance culture. London: Nicholas Saunders, 1995.
Copyright © 1998 by the American Academy of Neurology
From the Department of Neurology (Drs. Bolla and Ricaurte), Johns Hopkins Medical Institutions, Baltimore; and the Unit on Anxiety (Dr. McCann), Biological Psychiatry Branch, National Institute of Mental Health, Bethesda, MD. Supported by National Institutes of Health (NIH) grants PHS R01 DA06275, DA05707, DA05938, DA10217, and K02 DA00206 (G.A.R.); by National Institute of Mental Health-Intramural Research Program support (U.D.M.); and by a General Clinical Research Center grant (M01 RR02719, National Center for Research Resources, NIH) to Johns Hopkins Bayview Medical Center, Baltimore, MD.
Received by Neurology May 7, 1998. Accepted in final form August 20, 1998.
Address correspondence and reprint requests to Dr. Karen I. Bolla, Johns Hopkins Bayview Medical Center,
Department of Neurology, 4940 Eastern Ave.,
Baltimore, MD 21224.

