On April 10, 2024, after 23 years of continuous service as the only program offering anonymous mail-in lab testing of controlled substances in the United States, DrugsData went on pause. Our lab was ordered by the DEA to halt analysis of DrugsData samples and re-apply for our exception to the rules that disallow anonymous testing and the shipping of samples of possible controlled substances through the mail without a DEA license and specialized government forms.

As of September 24, 2024 we don’t yet know what caused this situation or have any real understanding about when or whether Erowid Center’s DrugsData project will be able to re-start. We’re disappointed in how long the process is taking, but we continue to be hopeful!
It is our express long-term goal for DrugsData to catalyze a shift toward allowing more labs across the United States to anonymously analyze samples from the public. The DEA is notoriously private about their internal processes, more so than many other federal agencies. They did tell us that they are trying to create a process for re-approving our project that they have not previously had, which adds to our hope.
One complication is that it is is our partner lab that has the DEA license, approval, and waiver of 21 CFR 1305.03(c), not Erowid Center. So Erowid isn’t in the ideal position to complain or press for answers. We made the decision to remain hopeful and not say anything publicly that would prompt public pressure that might annoy DEA officials. We are aware that our response to this situation could endanger our lab’s long-term relationship with the DEA, which we don’t want. A good-faith relationship is required for any controlled-substance testing lab to exist. If we do this wrong, we endanger the lab (our long-time partner), not Erowid Center.
Since early April, we’ve told the public that the project is going through “administrative red tape”, which summarizes the situation without going into too many details. But now that our annual September Drive is underway, we can no longer simply demur. Many Erowid supporters obviously have questions about what’s going on with our DrugsData project. So this is the first time we’re describing in more detail what has been going on.
We don’t think the program was stopped because of any suspicion of criminal activity or investigation into DrugsData. The implicit rules are that we do not violate the spirit or letter of the 1973-1974 regulation covering this topic. You can find the full entry (assembled into a PDF with the first page being the scanned pages of the Federal Register, and then the rest being an easier-to-read version of the same text) here:
https://www.erowid.org/freedom/law/federal_register/federal_register_anonymous_testing_rule_1974.pdf
The rule became binding in 1974. It’s a little long, but here is a very relevant section:
(f) “The delivery of such substances to a registered analytical laboratory, or its agent approved by DEA, from an anonymous source for the analysis of the drug sample: Provided, The laboratory has obtained a written waiver of the order form requirement from the Regional Director of the Region in which the laboratory is located, which waiver may be granted upon agreement of the laboratory to conduct its activities in accordance with Administration guidelines.”
The waiver mentioned in that paragraph is the waiver that our lab has received three times over the past 30+ years. The first was in 1988, the second was when we started EcstasyData in 2001, and the third was when the lab changed ownership in 2016. During the previous two re-approvals, the process took about six weeks, during which anonymous testing was not halted.
In contrast, over five months have passed since the latest application for re-approval was submitted.
Our hope is that DEA will see that we’re involved in important research projects designed to monitor street drugs and offer health agencies more insight into street drugs and the opioid crisis. At least one state Department of Public Health believes that our work, as part of a large team, has provided on-the-ground benefit to health care providers and end users. County officials continue to value the lab validation of on-site testing technologies, as well as the direct access their staff has to the results from their area, and from around the United States.
The pause on DrugsData testing is hurting research. It is actually causing harm to real people who are less able to learn about the contaminants in the drugs they consume. Stopping our project also hurts medical professionals and city-, county-, and state-level efforts to reduce harm through drug supply monitoring.
Other groups in the United States are doing lab drug checking as part of research projects and street drug harm reduction, many of which we collaborate with. Sadly, none accept samples from the public by mail. We like these groups a lot and would like to promote them, but in the context of describing our DEA issues, we’d prefer to not mention their names. We’ll save that for another update. The progress towards ubiquitous local drug checking in the United States over the last five years has been absolutely amazing, and we’re glad to continue to play a role in it, even while our own project is in limbo.
Thanks to everyone for their support of DrugsData and Erowid Center, and your patience during this long process.

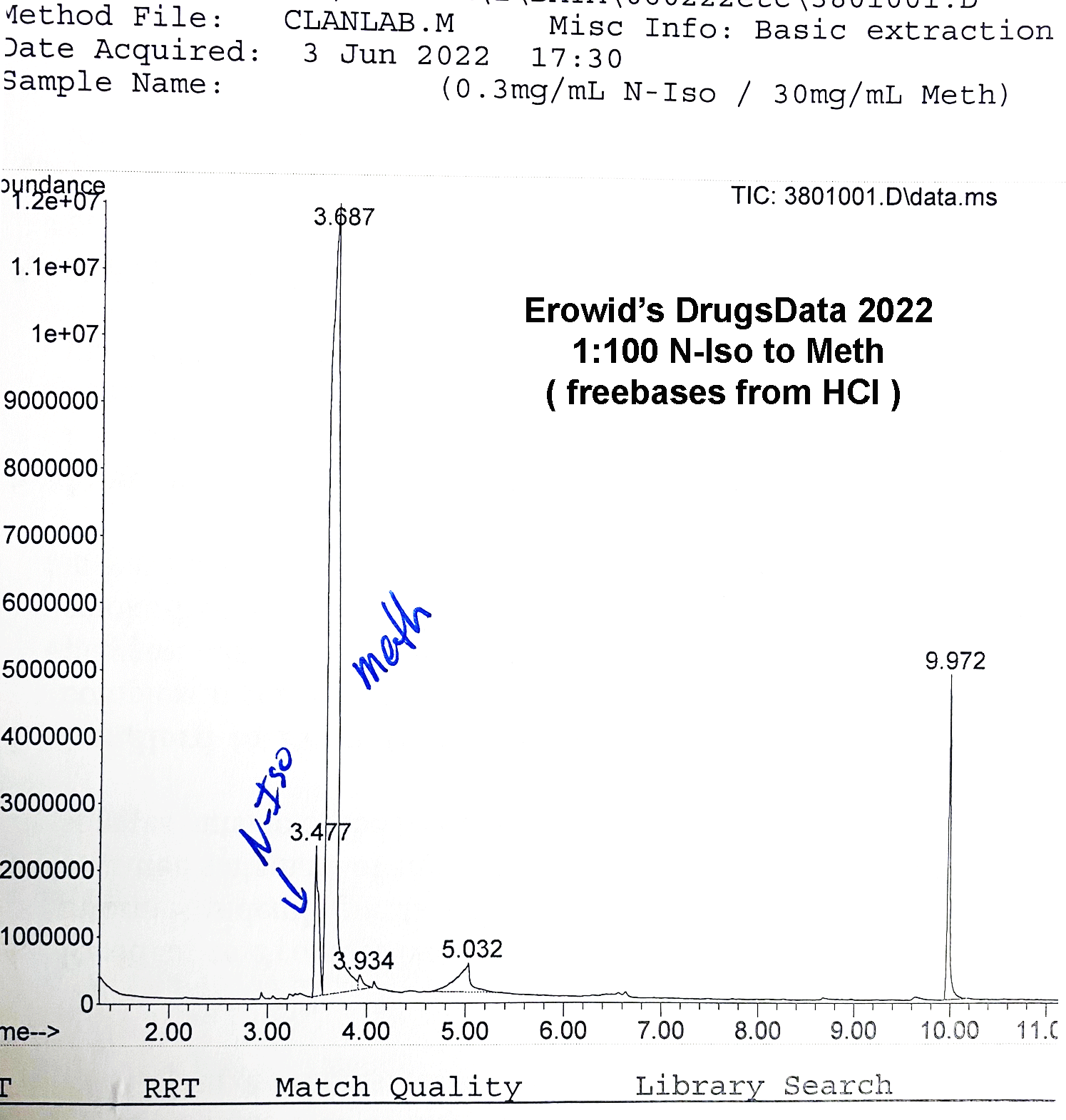

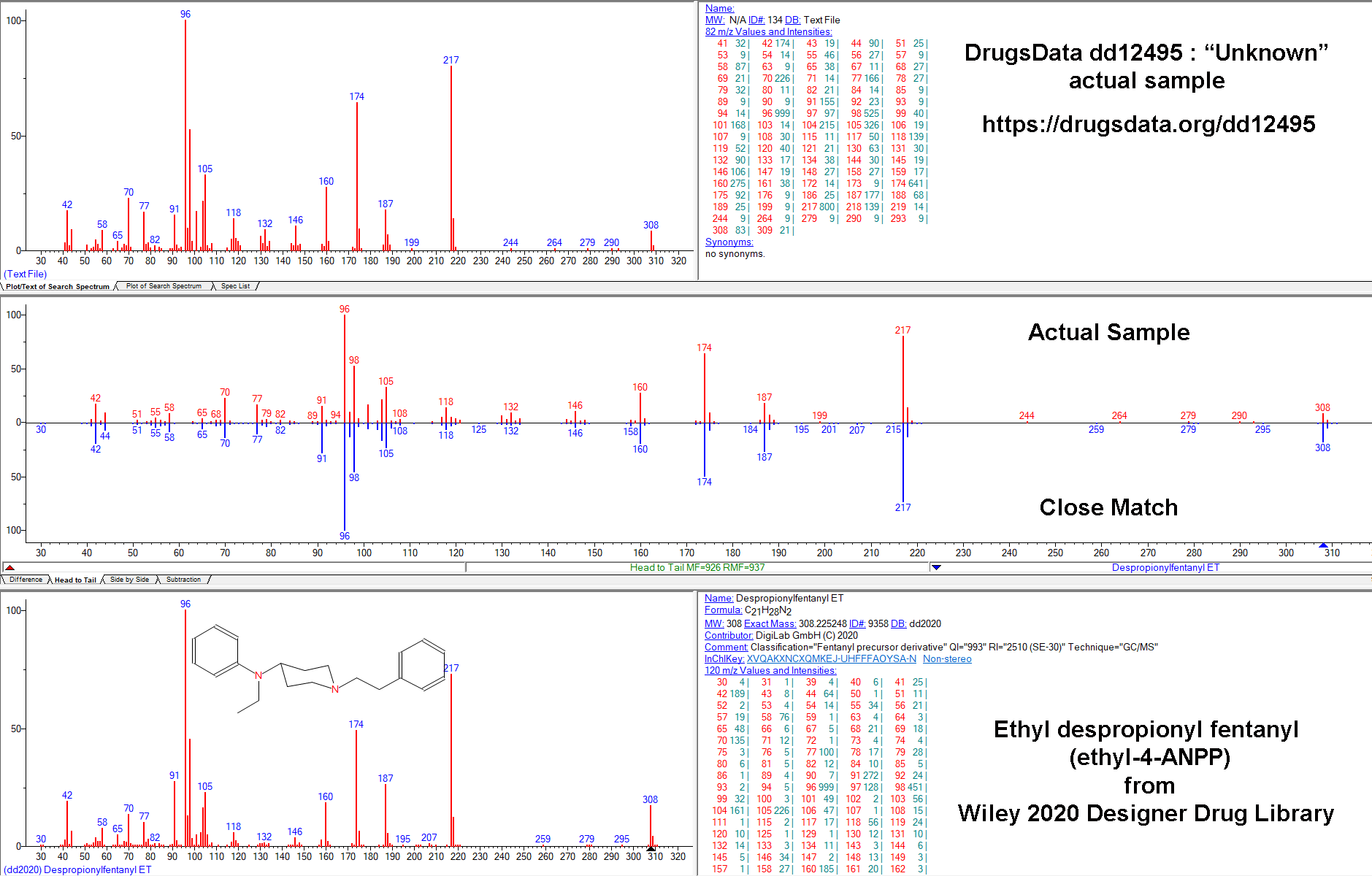
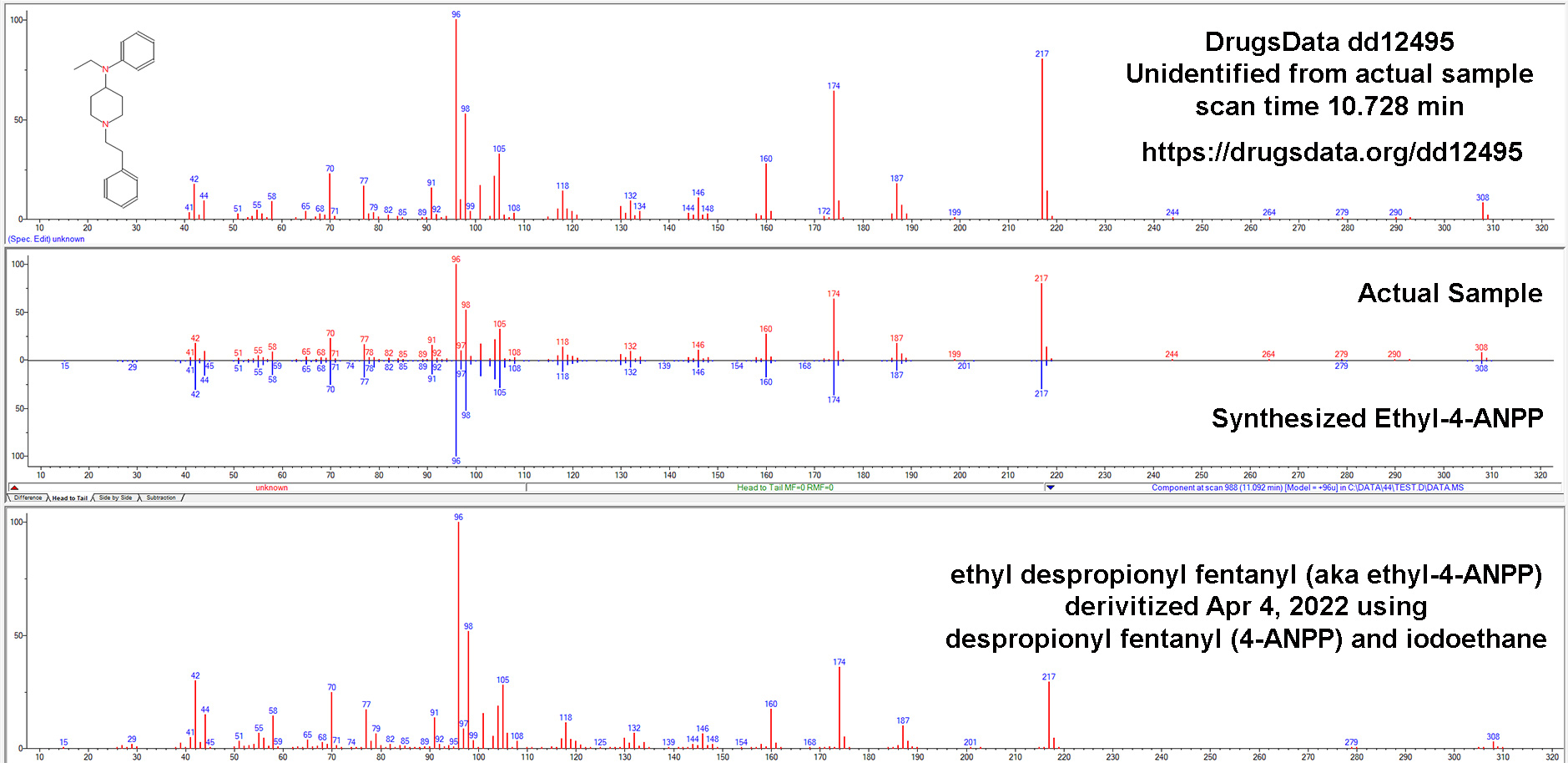



 cassette / dip card
cassette / dip card