
#46 2C-T-13
2,5-DIMETHOXY-4-(2-METHOXYETHYLTHIO)PHENETHYLAMINE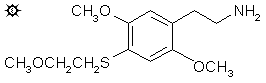
|
| [3D .mol structure] |
A mixture of 10 g POCl3 and 10 g N-methylformanilide was heated for 10 min on the steam bath. To this claret-colored solution was added 6.16 g of 2,5- dimethoxyphenyl 2-methoxyethyl sulfide. There was an immediate exothermic reaction and gas evolution. The mixture was heated for 15 min on the steam bath, at which time there was no starting sulfide present by TLC. This was then added to 500 mL of well-stirred warm H2O (pre-heated to 55 °C) and the stirring continued until only a thin oily phase remained. This was extracted with CH2Cl2, the extracts were combined, and the solvent removed under vacuum. The residue was extracted with 5 sequential 20 mL portions of boiling hexane which deposited crystals on cooling. Filtering gave a total of 4.12 g crystalline solids. Recrystallization from MeOH gave a poor yield of a cream-colored crystal with a mp of 68-69 °C. A more efficient purification was achieved by distillation (155-168 °C at 0.3 mm/Hg) yielding 3.50 g of 2,5-dimethoxy-4-(2-methoxyethylthio)benzaldehyde as a pale yellow solid, with a mp of 67-68 °C. A faster moving (by TLC) trace component with an intense fluo-rescence persisted throughout the entire purification scheme, and was still present in the analytical sample. Anal. (C12H16O4S) C,H.
To a solution of 3.41 g 2,5-dimethoxy-4-(2-methoxyethylthio)benzaldehyde in 50 g of nitromethane there was added 0.11 g of anhydrous ammonium acetate, and the mixture was heated on the steam bath for 2 h, at which time the starting aldehyde had largely disappeared by TLC (silica gel plates with CH2Cl2 as the developing solvent) and a faster moving nitrostyrene product was clearly visible. The clear orange solution was stripped of the excess nitromethane under vacuum producing a yellow oil that crystallized yielding 3.97 g of a yellow solid with a mp of 99-104 °C. Recrystallization of a small sample from MeOH produced (when dry) yellow electrostatic crystals of 2,5-dimethoxy-4-(2-methoxyethylthio)-beta-nitrostyrene with a mp of 107 °C sharp. From IPA the product is a burnished gold color with the mp 106-107 °C. Anal. (C13H17NO5S) C,H.
A solution of LAH (40 mL of a 1 M solution in THF) was cooled, under He, to 0 °C with an external ice bath. With good stirring there was added 1.05 mL 100% H2SO4 dropwise, to minimize charring. This was followed by the addition of 3.07 g 2,5-dimethoxy-4-(2-methoxyethylthio)-beta-nitrostyrene in small portions, as a solid, over the course of 10 min. There was a considerable amount of gas evolved, and a little bit of charring. After a few min further stirring, the temperature was brought up to a gentle reflux on the steam bath, and then all was cooled again to 0 °C. The excess hydride was destroyed by the cautious addition of 8 mL IPA followed by 3 mL 15% NaOH which gave the reaction mixture a curdy white granular character. The reaction mixture was filtered, the filter cake washed with THF, and filtrate and washes were stripped of solvent under vacuum providing about 3 g of a pale amber oil. This was dissolved in about 40 mL CH2Cl2 and extracted with 200 mL dilute H2SO4 in three portions. All of the color remained in the organic phase. The pooled aqueous extracts were washed with CH2Cl2, then made basic with 25% NaOH, extracted with 3x75 mL CH2Cl2, and the combined extracts pooled and stripped of solvent under vacuum. The 2 g pale yellow oily residue was distilled at 155-165 °C at 0.2 mm/Hg to give 1.23 g of a clear white oil. This was dissolved in IPA, neutralized with concentrated HCl, and diluted with anhydrous Et2O to produce crystals of 2,5-dimethoxy-4-(2-methoxyethylthio)phenethylamine hydrochloride (2C-T-13). After filtration, washing with Et2O, and air drying, this white crystalline product weighed 0.89 g.
DOSAGE: 25 - 40 mg.
DURATION: 6 - 8 h.
QUALITATIVE COMMENTS: (with 25 mg) I felt it was somewhat noisy as we went into the experience. This noisiness lasted only about an hour, then stopped. At the peak, which seemed to be at about 1 to maybe 1.5 hours, some eyes-closed visuals appeared. There was a white field with colored visuals, at times geometric in shape. These eye-closed images were pleasant and I enjoyed them when I did not concern myself with, or listen to, the conversation. There was an eyes-open change in color, the ivy became a little lighter or maybe a little stronger in color. I'm not sure which. I felt there was a gradual diminishing of activity (whatever that undefined activity was) starting at 2 to 2.5 hours, and coming close to baseline at 6 PM. The descent was pleasant and I would say pleasurable. The experience did not lead to any confusion which I sometimes notice in other experiences. There was no problem with anorexia. We ate constantly during the experience. The grapes and other fruit were lovely. This is one of the few times I would say that I would try a higher dose. Maybe 30 or 33 milligrams. I suspect the experience would be similar, with just a heightened peak at 1 hour and perhaps a little more body effect. It may well be one to try with one's wife.
(with 28 mg) There was a strange, disturbing twinge exactly eight minutes after starting this, that asked me, `Should I have done this?' I answered, `Yes' and the twinge disappeared. And then there was nothing until the expected time of development, at a half hour when I felt a light head and slight dizziness. There was a solid plus two for a couple of hours. I paid careful attention for auditory oddities that I had noted before, but they were not there. In an earlier trial (with 20 milligrams) the radio had the sound of being located in the outdoors with the sounds coming through the wall and into the room where I was. I was at a neutral baseline at about seven hours.
(with 35 mg) There was a quiet climb, but it was marred with some tummy unquiet, and an annoying persistence of diarrhea. I was very impressed with eyes-closed patterning, which seemed to do its own thing independently of the music. I was clearly up to a +++, but there was a feeling that as soon as it got there it started to go away again. There was no there, there. Yet there were a couple of touches of introspection, of seriousness which I had to respect.
(with 40 mg) There were four of us, and the entry was individual for each of us. Two of us were nauseous. One volunteered a statement, almost a confession, of too much food and drink in the immediate past. One of us needed his cigarette right now, and then he saw that he was killing himself, and he swore off. Don't know if it will last, however. At the two and a half hour point there is a consensus that this has gone its route and will lose its impact, so three of us decided to supplement on 2C-T-2. Six milligrams proves to be a little light so, some four hours later, we each took another six milligrams. Excellent. In a while we discoved that we were very hungry, and food tasted marvelous. Headaches acknowledged in the early evening, but the extension from T-13 to T-2 seemed to be absolutely correct. And as of the next day, the non-smoker was still a non-smoker.
EXTENSIONS AND COMMENTARY: Most of the synthetic adventures of putting a basic something aways out from the benzene ring, at the four-position, have involved subtle things such as unsaturated bonds or three-membered rings. This was the first try with the actual use of a different atom (an oxygen). What about other heteroatoms such as sulfur or nitrogen or silicon or phosphorus, or some-such?
The sulfur counterpart of 2C-T-13 was named 2C-T-14, and was immediately launched. The reaction of 2,5-dimethoxythiophenol and KOH with 2-methyl-thioethyl chloride in hot MeOH gave 2,5-dimethoxyphenyl 2-methylthioethyl sulfide as a white oil (boiling point of 140-160 °C at 0.3 mm/Hg). This underwent a normal Vilsmeier reaction (phosphorous oxychloride and N-methylformanilide) to give 2,5-dimethoxy-4-(2-methylthioethylthio)benzaldehyde with a melting point of 64-64.5 °C from MeOH. This, in nitromethane containing a little ammonium acetate, was heated on the steam bath for 10 hours and worked up to give an excellent yield of 2,5-dimethoxy-4-(2-methylthioethylthio))-beta-nitrostyrene as garish orange-red "Las Vegas" colored crystals from acetonitrile, with a melting point of 126-127 °C. And as of the moment, this is sitting on the shelf waiting to be reduced to the target compound 2,5-dimethoxy-4-(2-methylthioethylthio)phenethylamine hydrochloride, or 2C-T-14. Will it be active? I rather suspect that it will be, and I'll bet it will be longer-lived than the oxygen model, 2C-T-13.
| [ |
[Main Index] | [Forward |

