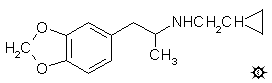
#104 MDCPM
CYCLOPROPYLMETHYL-MDA; 3,4-METHYLENEDIOXY-N-CYCLOPROPYLMETHYLAMPHETAMINE
SYNTHESIS: A solution of 9.4 g cyclopropylmethylamine hydrochloride in
30 mL MeOH was treated with 1.8 g 3,4-methylenedioxyphenylacetone (see
under MDMA for its preparation) followed by 0.5 g sodium
cyanoborohydride. Concentrated HCl was added as needed to keep the pH
constant at about 6. After several days stirring, the reaction
mixture was added to H2O, acidified with HCl, and washed with 2x100 mL
CH2Cl2. The aqueous phase was made basic with 25% NaOH, and extracted
with 3x150 mL CH2Cl2. Removal of the solvent from these extracts
under vacuum yielded 2.8 g of a crude product which, on distillation
at 90-100 °C at 0.1 mm/Hg, yielded 0.4 g of a clear white oil. This
was dissolved in a small amount of IPA, neutralized with a few drops
of concentrated HCl, and diluted with anhydrous Et2O to the point of
turbidity. There was obtained a small yield of crystalline
3,4-methylenedioxy-N-cyclopropylmethylamphetamine hydrochloride
(MDCPM) which was filtered off, Et2O washed and air dried. The mp was
218-220 °C, with extensive darkening just prior to melting. Anal.
(C14H20ClNO2) N.
DOSAGE: greater than 10 mg.
DURATION: unknown.
EXTENSIONS AND COMMENTARY: The record of the tasting assay of this
compound is pretty embarrassing. The highest level tried was 10
milligrams, which showed no hint of activity. But in light of the
rather colorful activities of other cyclopropylmethyl things such as
CPM and 2C-T-8 , this compound might someday warrant reinvestigation.
It is a certainty that the yield could only be improved with a careful
resynthesis.