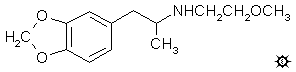
#112 MDMEOET
N-METHOXYETHYL-MDA; 3,4-METHYLENEDIOXY-N-(2-METHOXYETHYL)AMPHETAMINE
SYNTHESIS: A crude solution of methoxyethylamine hydrochloride was
prepared from 17.7 g methoxyethylamine and 20 mL concentrated HCl with
all volatiles removed under vacuum. This was dissolved in 75 mL MeOH
and there was added 4.45 g of 3,4-methylenedioxyphenylacetone (see
under MDMA for its preparation) followed by 1.3 g sodium
cyanoborohydride. Concentrated HCl in MeOH was added as required to
maintain the pH at about 6 as determined with external, dampened
universal pH paper. About 4.5 mL were added over the course of 5
days, at which time the pH had stabilized. The reaction mixture was
added to 400 mL H2O and made strongly acidic with an excess of HCl.
After washing with 2x100 mL CH2Cl2 the aqueous phase was made basic
with 25% NaOH, and extracted with 4x75 mL CH2Cl2. Removal of the
solvent under vacuum yielded 6.0 g of an amber oil that was distilled
at 110-120 °C at 0.2 mm/Hg. There was obtained 4.7 g of a
crystal-clear white oil that was dissolved in 30 mL IPA and
neutralized with 45 drops of concentrated HCl producing a heavy mass
of spontaneous crystals that had to be further diluted with IPA just
to be stirred with a glass rod. These were diluted with 200 mL of
anhydrous Et2O, removed by filtration, and washed with additional
Et2O. After air drying there was obtained 4.9 g of
3,4-methylenedioxy-N-(2-methoxyethyl)amphetamine hydrochloride
(MDMEOET) with a mp of 182.5-183 °C. Anal. (C13H20ClNO3) N.
DOSAGE: greater than 180 mg.
DURATION: unknown.
EXTENSIONS AND COMMENTARY: This is another example of the replacement
of a neutral atom out near the end of a chain, with a more basic and a
more polar one. MDMEOET would be called an isostere of MDBU in that
it has the same shape, with a methylene unit (the CH2) replaced by an
oxygen atom. No activity turned up with either compound, so nothing
can be learned from this particular example of change of polarity.