
#126 METHYL-DMA
DMMA; 2,5-DIMETHOXY-N-METHYLAMPHETAMINE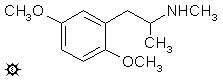
|
| [3D .mol structure] |
DOSAGE: above 250 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 250 mg) There is a slight paresthesia at about 45 minutes, an awareness on the surface of the skin as if I had been touched by a cold draft of air. But nothing more. At three hours, I am completely out, if I was ever in. In the evening I assayed 120 milligrams of MDMA, and it barely produced a threshold effect, so the two materials might be seeing one another.
EXTENSIONS AND COMMENTARY: This is a difficult compound to pin down in the anthology of drugs. For some reason it has intrigued several independent, quiet researchers, and I have accumulated a number of interesting reports over the years. One person told me that he had felt nothing at up to 60 milligrams. Another had found a threshold at 50 milligrams, and had complete and thorough experiences at both 150 and 200 milligrams. Yet another person described two incidents involving separate individuals, with intravenous administrations of 0.2 mg/Kg, which would be maybe 15 or 20 milligrams. Both claimed a real awareness in a matter of minutes, one with a tingling in the genitalia and the other with a strange presence in the spine. Both subjects reported increases in body temperature and in blood pressure. Apparently the effects were felt to persist for many hours.
There is an interesting, and potentially informative, convergence of the metabolite of one drug with the structure of another. Under 4-MA, mention was made of a bronchodilator that has been widely used in the treatment of asthma and other allergenic conditions. This compound, 2-methoxy-N-methylamphetamine is known by the generic name of methoxyphenamine, and a variety of trade names with Orthoxine (Upjohn) being the best known. The typical dosage of methoxyphenamine is perhaps 100 milligrams, and it may be used several times a day. It apparently produces no changes in blood pressure and only a slight cardiac stimulation. And one of the major metabolites of it in man is the analogue with a hydroxyl group at the 5-position of the molecule. This phenolic amine, 5-hydroxy-2-methoxy-N-methylamphetamine is just a methyl group away from METHYL-DMA; it could either be methylated to complete the synthesis, or METHYL-DMA could be demethylated to form this phenol. There is plentiful precedent for both of these reactions occuring in the body. It is always intriguing when drugs which show distinctly different actions can, in principle, intersect metabolically at a single structure. One wonders just what the pharmacology of that common intermediate might be.
Three additional N-methylated homologues of known psychedelics warrant mention, but do not really deserve separate recipes. This is because they have had only the most cursory assaying, which I have learned about by personal correspondence. All three were synthesized by the reduction of the formamide of the parent primary amine with LAH. METHYL-TMA (or N-methyl-3,4,5-trimethoxyamphetamine) had been run up in several trials to a maximum of 240 milligrams, with some mental disturbances mentioned only at this highest level. METHYL-TMA-2 (or N-methyl-2,4,5-trimethoxyamphetamine) had been tried at up to 120 milligrams without any effects. METHYL-TMA-6 (or N-methyl-2,4,6- trimethoxyamphetamine) had been tried at up to 30 milligrams and it, too, was apparently without effects. These are reports that I have heard from others, but I have had no personal experience with them. Those that I can describe from personal experience are entered separately as recipes of their own. And there are many, many other N-methyl homologues which have been prepared and characterized in the literature, and have yet to be tasted. So far, however, the only consistent thing seen is that, with N-methylation, the potency of the psychedelics is decreased, but the potency of the stimulants appears to be pretty much maintained.
| [ |
[Main Index] | [Forward |

