
#144 SB
SYMBESCALINE; 3,5-DIETHOXY-4-METHOXYPHENETHYLAMINE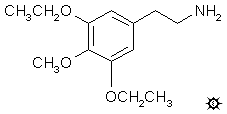
|
| [3D .mol structure] |
A solution of 7.6 g 2,6-diethoxyphenol in 40 mL MeOH was treated with 4.9 g of a 40% aqueous solution of dimethylamine followed by 3.6 g of a 40% aqueous solution of formaldehyde. The mixture was heated 1 h on the steam bath, and all volatiles were removed under vacuum. The residual dark oil was dissolved in 36 mL IPA and 10.3 g of methyl iodide was added. There was spontaneous heating, and the deposition of fine white solids. After standing for 10 min, these were removed by filtration, and the filter cake washed with more IPA. The crude product was freed from solvent (air dried weight, 1.7 g) and dissolved in 7 mL hot H2O. To this hot solution there was added 1.7 g sodium cyanide which slowly discharged the color and again deposited flocculant white solids. After cooling, these were removed by filtration, washed with H2O, and after thorough drying the isolated 3,5-diethoxy-4-hydroxyphenylacetonitrile weighed 0.5 g and had a mp of 107.5-108.5 °C. Anal. (C12H15NO3) C,H.
To a solution of 2.1 g 3,5-diethoxy-4-hydroxyphenylacetonitrile in 20 mL anhydrous acetone, there was added 30 mg triethyldecylammonium iodide, 4.6 g methyl iodide, and finally 2.3 g powdered anhydrous K2CO3. This mixture was held at reflux for 5 h. The reaction mixture was quenched with 200 mL acidified H2O and extracted with 3x75 mL CH2Cl2. The extracts were pooled, washed with 2x75 mL 5% NaOH, and finally once with dilute HCl. The solvent was removed under vacuum, and the residue distilled at 110-115 °C at 0.3 mm/Hg to provide 3,5-diethoxy-4-methoxyphenylacetonitrile as a solid. This weighed 1.3 g and had a mp of 58-59 °C. Anal. (C13H17NO3) C,H.
To 30 mL of a 1 M solution LAH in THF that had been cooled to 0 °C with vigorous stirring, under a He atmosphere, there was added dropwise 0.78 mL of 100% H2SO4. When the addition was complete, there was added dropwise a solution of 1.3 g of 3,5-diethoxy-4-methoxyphenylacetonitrile in 10 mL anhydrous THF. The reaction mixture was brought to room temperature and stirred an additional 10 min, then refluxed on a steam bath for 1.5 h. After cooling to room temperature the excess hydride was destroyed by the addition of about 2 mL IPA, followed by sufficient 15% NaOH to make the reaction basic to external pH paper and to render the aluminum oxides white and filterable. These were removed by filtration, the filter cake was washed with IPA, then the filtrate and washes were combined. The solvents were removed under vacuum and the residue dissolved in dilute H2SO4. This was washed with 2x75 mL CH2Cl2, the aqueous phase made basic with 5% NaOH, and extracted with 3x75 mL CH2Cl2. The extracts were pooled, the solvent removed under vacuum, and the residue distilled at 120-140 °C at 0.3 mm/Hg to yield 0.9 g of a white oil. This was dissolved in 4 mL of IPA and neutralized with concentrated HCl to an end-point determined by damp external pH paper. There was the immediate formation of solids which were removed by filtration and washed first with IPA and then with Et2O. This provided 1.0 g of 3,5-diethoxy-4-methoxyphenethylamine hydrochloride (SB) as white crystals, with a mp of 186-187 °C. Anal. (C13H22ClNO3) C,H.
DOSAGE: above 240 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 120 mg) There were no effects. Sleep that evening was strange, however, and I was fully awake at 4:00 AM, alert, and mentally restless. And there was a strange outburst of anger in the mid-morning. Might these be related to the material the previous day?
(with 240 mg) There was a slight chill that reminded me that I had taken symbescaline a half hour earlier. There was what might be called a vague threshold for about three hours, then nothing more. This material had a God-awful taste that lingers in the mouth far too long. If ever again, it will be in a gelatin capsule.
EXTENSIONS AND COMMENTARY: It must be concluded that SB is "probably" not active. There was no convincing evidence for much effect at levels that would clearly be active for mescaline. This is the kind of result that puts some potentially ambiguous numbers in the literature. One cannot say that it is inactive, for there might well be something at 400 or 800 or 1200 milligrams. But since it has been tried only up to 240 milligrams, I have used the phrase that the activity is greater than 240 milligrams. This will be interpreted by some people as saying that it is active, but only at dosages higher than 240 milligrams. What is meant, is that there was no activity observed at the highest level tried, and so if it is active, the active dose will be greater than 240 milligrams, and so the potency will be less than that of mescaline. However you phrase it, someone will misinterpret it.
| [ |
[Main Index] | [Forward |

